
2-(4-Nitrophenylamino)ethyl methanesulfonate synthesis
- Product Name:2-(4-Nitrophenylamino)ethyl methanesulfonate
- CAS Number:100418-51-7
- Molecular formula:C9H12N2O5S
- Molecular Weight:260.27
Yield:100418-51-7 99%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at 0 - 20;
Steps:
5.1
Compound 17 (15 g, 82.33 mmol) and diisopropylethylamine (27 mL, 164 mmol) were dissolved in dichloromethane (150 mL), to which methanesulfonylchloride (9.5 mL) was slowly loaded at 0° C., followed by stirring at room temperature for 2 hours. Upon completion of the reaction, dichloromethane (800 mL) was added to the reaction solution. The reactant was washed with sodium bicarbonate solution (500 mL) and concentrated under reduced pressure to give yellow solid compound 18 (22 g, 82.00 mmol, 99%).1H NMR (400 MHz, chloroform-d1) δ 8.12 (d, J=9.2 Hz, 2H), 6.60 (d, J=9.2 Hz, 2H), 4.45 (t, J=5.6 Hz, 2H), 3.63 (t, J=5.6 Hz, 2H), 3.06 (s, 3H)LCMS: 183 (M+H+) to C8H10N2O3S
References:
US2011/112083,2011,A1 Location in patent:Page/Page column 19
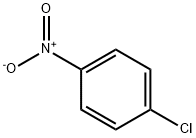
100-00-5
54 suppliers
$14.14/5gm:

100418-51-7
8 suppliers
inquiry
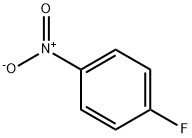
350-46-9
522 suppliers
$10.60/1gm:

100418-51-7
8 suppliers
inquiry
![2-[(4-NITROPHENYL)AMINO]ETHANOL](/CAS/GIF/1965-54-4.gif)
