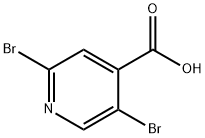
2,5-DIBROMOISONICOTINIC ACID synthesis
- Product Name:2,5-DIBROMOISONICOTINIC ACID
- CAS Number:942473-59-8
- Molecular formula:C6H3Br2NO2
- Molecular Weight:280.9
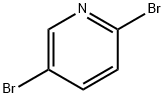
624-28-2
729 suppliers
$5.00/5g

124-38-9
129 suppliers
$175.00/23402

942473-59-8
69 suppliers
$20.00/1g
Yield:-
Reaction Conditions:
Stage #1:2,5-dibromopyridine with n-butyllithium;diisopropylamine in tetrahydrofuran at -80 - -70; for 1.5 h;Inert atmosphere;
Stage #2:carbon dioxide in tetrahydrofuran at -70; for 0.5 h;
Steps:
12.1 Step 1
Step 1
A 20-L 4-necked round-bottom flask purged and maintained under a N2 atmosphere was charged with a solution of diisopropylamine (582 g, 5.76 mol, 1.05 equiv) in THF (4.2 L), followed by the dropwise addition over 30 min of n-BuLi (2.4 M, 2.4 L, 1.05 equiv) with stirring at -30° C.
The resulting solution was stirred at -30° C. for 30 min then cooled to -80° C.
To the LDA solution was added dropwise over 1 h with stirring a solution of 2,5-dibromopyridine (1.3 Kg, 5.49 mol, 1.00 equiv) in THF (5.2 L).
The resulting solution was stirred at -70° C. for 30 min.
To the mixture was added CO2 (dry ice) (1267 g, 28.80 mol, 5.00 equiv) in several batches at -70° C.
The resulting solution was stirred at -70° C. for 30 min, quenched by the addition of 5 L of water at -70° C., concentrated in vacuo and extracted with 3*4 L of EtOAc.
The pH value of the aqueous layer was adjusted to 3-4 with HCl (12 mol/L).
The precipitate was collected by filtration to afford 2,5-dibromoisonicotinic acid (A-2) white solid.
References:
Genentech, Inc.;Array BioPharma Inc.;Blake, Jim;Chen, Huifen;Chicarelli, Mark;Gaudino, John;Gazzard, Lewis;Kintz, Sam;Mohr, Pete;Robarge, Kirk;Schwarz, Jacob;Zhou, Aihe US2014/66453, 2014, A1 Location in patent:Paragraph 0270-0271