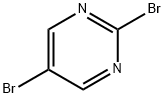
2,5-Dibromopyrimidine synthesis
- Product Name:2,5-Dibromopyrimidine
- CAS Number:32779-37-6
- Molecular formula:C4H2Br2N2
- Molecular Weight:237.88
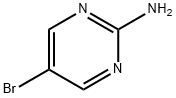
7752-82-1

32779-37-6
General procedure for the synthesis of 2,5-dibromopyrimidines from 2-amino-5-bromopyrimidines: Scheme 1. Preparation of the related pyran(mi)dihalides A-H. The key steps include: (a) Reaction using N-bromosuccinimide (NBS) and ammonium acetate (NH4OAc) in acetonitrile (MeCN) at room temperature for 5 min in 85-90% yield of pyridine (pyr); quantitative yield of pyrimidine (pym); (b) Pyridine: 1-12 h reflux reaction in acetonitrile with aldehyde (RCHO) and sodium cyanoborohydride (Na(CN)BH3) (82% yield, R = C5Hn); pyrimidine: overnight reaction at room temperature in tetrahydrofuran (THF) using sodium hydride (NaH) and alkyl halide (R1) (85% yield, R = Me); (c) Pyridine in 77-83% yield; pyrimidine in 30-40% yield using trimethylbenzylammonium bromide (Me3(Bn)NBr) and tert-butylnitrite (t-BuONO) in dibromomethane (CH2Br2), room temperature reaction overnight; (d) Pyrimidine: 80-85% yield in dichloromethane (CH2Cl2) using hydriodic acid (HI), reacted at 0 °C; (e) i. In water (H2O), using sodium hydroxide (NaOH) and bromine (Br2), reacted at room temperature in 50-60% yield; ii. In phosphorus oxychloride (POCl3) and diethylaniline (PhNEt2), reacted at reflux for 4 hrs in 75-85% yield; iii. In dichloromethane (DCM), using hydriodic acid (HI), reacted at 0°C in 80-85% yield; (f) in alcohol (ROH), using sodium (Na), 1-12 h reaction at room temperature, quantitative yield; (g) Overnight reaction at room temperature using alkyl zinc iodide (RZnI) and dichlorobis(triphenylphosphine)palladium (Cl2Pd(PPh3)2) in N,N-dimethylformamide (DMF)/tetrahydrofuran (THF) in 72% (R = C6H13) yield of pyridine (bromo-substituted) and 81% (R = C6H13) yield of pyrimidine (iodo-substituted); (h) Quantitative yields in acetonitrile using alkynes, cuprous iodide (CuI), dichlorobis(triphenylphosphine)palladium, and triethylamine (Et3N), reacted for 1-12 h at room temperature. Pyrimidinyl bromides were prepared in a similar manner starting from the bromination of 2-aminopyrimidine. N-alkylation could not be achieved by reductive amination (probably due to the reduced nucleophilicity of the amine), but was achieved using sodium hydride and appropriate alkyl halides (B). Non-aqueous diazotization/halogenated deoxidation was used to prepare 5-bromo-2-halogenated pyrimidines, but in reduced yield relative to a similar reaction with 2-aminopyridine (again, possibly due to reduced nucleophilicity of the amine group). Alternatively, the 2-pyrimidinone can be used as a precursor to 5-bromo-2-halogenopyrimidines (Lutz, F.; Kawasaki, T.; Soai, K. Tetrahedron-Asymmetry 2006, 17, 486) or as a substrate for alkylation, yielding 5-bromo-2-alkoxypyrimidines (D) (Kokatla, H.P. Lakshman, M.K. Org. Lett. 2010, 12, 4478). Introduction of an alkynyl substituent at the 2-position (carried out satisfactorily under Sonogashira conditions), but alkylation using Negishi conditions was non-selective. Since bromine was competitively removed upon reduction of 2-alkynylpyrimidinyl bromides (F) to the corresponding 2-alkylpyrimidinyl bromides (H), we turned to 5-bromo-2-iodopyrimidines as precursors for the cross-coupling reaction, with significantly improved selectivity and yield.

7752-82-1
428 suppliers
$13.00/25g

32779-37-6
193 suppliers
$9.00/1g
Yield:32779-37-6 73.2%
Reaction Conditions:
Stage #1: 5-bromo-2-aminopyrimidinewith hydrogen bromide;bromine in water at 0 - 4;Inert atmosphere;
Stage #2: with sodium nitrite in water at 0 - 4; for 3 h;
Steps:
2 Example 2
Under nitrogen protection, 34.8 g (0.2 mol) of 2-amino-5-bromopyrimidine and 180 mL of 48% HBr aqueous solution were put into the reaction flask, and 48.0 g (0.3 mol) of bromine was added at 0-4°C to form a yellow suspension. 55.2 g (0.4 mol) of a 50% aqueous solution of sodium nitrite was added dropwise at a temperature of 0-4 °C. After the addition, the mixture was stirred at 0-4° C. for 3 h, the raw material was less than 3% detected by HPLC, and then poured into 100 mL of ice. Neutralize with 30% aqueous sodium hydroxide solution and adjust pH=10-11, and extract with dichloromethane, the organic phase is concentrated to no flow, and then recrystallized with 100 mL ethyl acetate: petroleum ether=1:4 to obtain a white solid 2,5-dibromopyrimidine 34.83 g, yield 73.2%,
References:
CN113683571,2021,A Location in patent:Paragraph 0031-0033
38353-09-2
404 suppliers
$5.00/5g

32779-37-6
193 suppliers
$9.00/1g
32779-36-5
634 suppliers
$5.00/5g

32779-37-6
193 suppliers
$9.00/1g
38353-06-9
333 suppliers
$15.00/1g

32779-37-6
193 suppliers
$9.00/1g

38353-06-9
333 suppliers
$15.00/1g

32779-37-6
193 suppliers
$9.00/1g