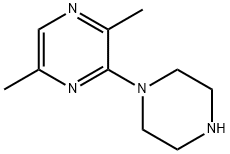
2,5-dimethyl-3-piperazin-1-ylpyrazine synthesis
- Product Name:2,5-dimethyl-3-piperazin-1-ylpyrazine
- CAS Number:59215-42-8
- Molecular formula:C10H16N4
- Molecular Weight:192.26
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

59215-42-8
15 suppliers
$95.00/200 μmol
Yield: 45%
Reaction Conditions:
with ammonium formate;palladium 10% on activated carbon in methanolHeating / reflux;
Steps:
16 Preparation 16: 2, 5-dimethyl-3-piperazin-1-ylpyrazine
Preparation 16: 2, 5-dimethyl-3-piperazin-1-ylpyrazine To a solution of 2, 5-dimethyl-3- [4- (phenylmethyl) piperazin-1-yl] pyrazine (Preparation 17,1. 14 g, 4.04 MMOL) in methanol (80 ML) was added ammonium formate (1.27 g, 20.2 MMOL) followed by palladium (10% w/w on carbon, 0.17 g) under a nitrogen atmosphere. The reaction mixture was heated under reflux, after 2.5 h, more ammonium formate (0.64 g, 10.1 MMOL) and palladium (0.06 g) were added. The reaction mixture was filtered through CELITEE washed with methanol and concentrated in vacuo. The crude product was purified by flash chromatography using silica gel eluting with methanol : dichloromethane (8: 92 and then 10: 90) and then methanol dichloromethane : 0.88 ammonia solution (10: 90: 1). The title compound was obtained as a white solid (0.35 g, 45%).
References:
PFIZER LIMITED;PFIZER INC. WO2004/72086, 2004, A2 Location in patent:Page 155

110-85-0
590 suppliers
$5.00/5 g
95-89-6
200 suppliers
$9.00/1g

59215-42-8
15 suppliers
$95.00/200 μmol