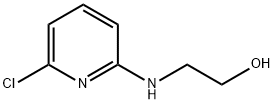
2-[(6-Chloro-2-pyridinyl)amino]-1-ethanol synthesis
- Product Name:2-[(6-Chloro-2-pyridinyl)amino]-1-ethanol
- CAS Number:29449-82-9
- Molecular formula:C7H9ClN2O
- Molecular Weight:172.61
2402-78-0

141-43-5
![2-[(6-Chloro-2-pyridinyl)amino]-1-ethanol](/CAS/GIF/29449-82-9.gif)
29449-82-9
The general procedure for the synthesis of 2-((6-chloropyridin-2-yl)amino)ethanol from 2,6-dichloropyridine and 2-aminoethanol was as follows: referring to the method of Example 67, 2-aminoethanol (0.82 g, 13.5 mmol) was added to a pyridine solution (10 mL) containing 2,6-dichloropyridine (2.0 g, 13.5 mmol) and the reaction was at room temperature The reaction was carried out at room temperature. Subsequently, the reaction mixture was heated to 100 °C and kept overnight. After completion of the reaction, the mixture was concentrated under vacuum to give a residue. The residue was dissolved in ethyl acetate, washed sequentially with water and brine, dried over anhydrous sodium sulfate, and finally the solvent was evaporated under vacuum to afford the target product 2-((6-chloropyridin-2-yl)amino)ethanol (2.3 g, 99% yield) as a white solid.

2402-78-0
513 suppliers
$6.00/25g

141-43-5
920 suppliers
$9.00/10g
![2-[(6-Chloro-2-pyridinyl)amino]-1-ethanol](/CAS/GIF/29449-82-9.gif)
29449-82-9
24 suppliers
$38.00/250mg
Yield:29449-82-9 99%
Reaction Conditions:
with pyridine at 20 - 100;
Steps:
67
Reference example 67: 2-(6-chloro-pyridin-2-ylaminoVethanol. 2-Arnino-ethanol (0.82 g, 13.5 mmol) was added to a solution of 2,6- dichloropyridine (2.0 g, 13.5 mmol) in pyridine (10 mL) at room temperature and then heated at 100 °C overnight The reaction mixture was concentrated in vacuo to obtain a residue which was dissolved in ethyl acetate. The solution was washed with water, brine, dried over anhydrous sodium sulfate and evaporated in vacuo to afford 2-(6- chloro-pyridin-2-ylamino)-ethanol (2.3 g, 99%) as a white solid.
References:
WO2008/62182,2008,A1 Location in patent:Page/Page column 128