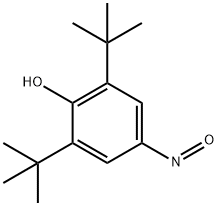
2,6-DI(TERT-BUTYL)-4-NITROSOPHENOL synthesis
- Product Name:2,6-DI(TERT-BUTYL)-4-NITROSOPHENOL
- CAS Number:955-03-3
- Molecular formula:C14H21NO2
- Molecular Weight:235.32
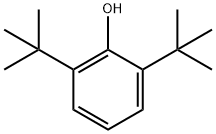
128-39-2
362 suppliers
$5.00/25g

955-03-3
23 suppliers
inquiry
Yield:955-03-3 91.7%
Reaction Conditions:
with acetic anhydride;NaNO2 in methanol;lithium hydroxide monohydrate at 15 - 30; for 0.5 h;Reagent/catalyst;Solvent;
Steps:
3.1 1. Preparation of 2,6-di-tert-butyl-4-nitrosophenol (Compound I)
2,6-di-tert-butylphenol (41.2 g, 0.2 mol) was weighed into a 1000 ml flask, and 200 ml of methanol was added.Add acetic anhydride (21g, 0.2mol), stir, until the solid disappears, put in a water bath;Slowly add a sodium nitrite solution at 15 ° C, including sodium nitrite (34.5.0 g, 0.5 mol) and80ml of water, exothermic reaction, after the addition is completed, yellow crystals are precipitated;Heating to 30 ° C for half an hour, adding liquid alkali to adjust the pH to 8-9; after half an hour of reaction, suction filtration with a suction filter funnel;Pour the liquid into a three-necked flask, add 12.5 g of concentrated sulfuric acid, and wash with yellow crystals;The yellow crystals were suction-dried and weighed, and the yield of the compound I was 91.7%.
References:
CN109651280,2019,A Location in patent:Paragraph 0046-0048; 0055-0057; 0064-0066
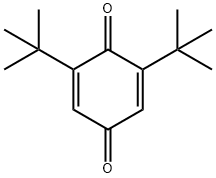
719-22-2
182 suppliers
$14.00/5g

955-03-3
23 suppliers
inquiry
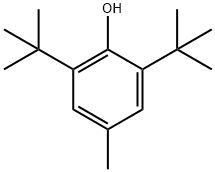
128-37-0
1188 suppliers
$5.00/25g

955-03-3
23 suppliers
inquiry