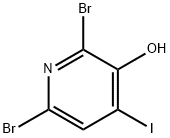
2,6-dibroMo-4-iodopyridin-3-ol synthesis
- Product Name:2,6-dibroMo-4-iodopyridin-3-ol
- CAS Number:478148-75-3
- Molecular formula:C5H2Br2INO
- Molecular Weight:378.79
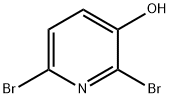
6602-33-1
114 suppliers
$14.00/250mg

478148-75-3
8 suppliers
inquiry
Yield:478148-75-3 92%
Reaction Conditions:
Stage #1: 2,6-dibromopyridin-3-olwith iodine;sodium hydrogencarbonate in water at 20; for 120 h;
Stage #2: with hydrogenchloride in water; pH=2;
Steps:
Preparation of 72Adapted from the method of Wishka et al, WO 2002/100857.71 (10.0 g, 39.5 mmol), sodium bicarbonate (12.0 g, 142.8 mmol) and iodine (12.4 g, 48.9 mmol) were combined in water (200 mL) and stirred at r.t. for 5 days. Excess iodine was then quenched by addition of sodium thiosulfate (12.0 g) and the pH was adjusted to 2 by addition of cone. HCl. The resultant precipitate was collected and purified twice by flash column chromatography (Si02, gradient elution from 100% pet. ether to 100% EtOAc), ground to a fine powder and dried in vacuo to afford 72 as a pale pink amorphous solid ([98% purity by 1 H NMR spectroscopy, where the remaining impurity was 71.Used without further purification.] 14.0 g, 36.6 mmol, 92%).
References:
WO2011/98776,2011,A1 Location in patent:Page/Page column 61
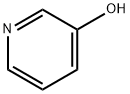
109-00-2
607 suppliers
$10.00/10g

478148-75-3
8 suppliers
inquiry