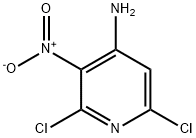
2,6-DICHLORO-3-NITRO-4-AMINOPYRIDINE synthesis
- Product Name:2,6-DICHLORO-3-NITRO-4-AMINOPYRIDINE
- CAS Number:2897-43-0
- Molecular formula:C5H3Cl2N3O2
- Molecular Weight:208
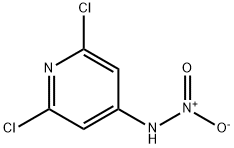
2587-03-3

2897-43-0
2,6-Dichloro-4-nitroaminopyridine (CAS: 2587-03-3, 1.66 g, 7.98 mmol) was used as a raw material, which was slowly added to 11 mL of concentrated sulfuric acid and stirred until completely dissolved. The reaction mixture was heated on a steam bath for 30 min. Upon completion of the reaction, the solution was cooled to room temperature and slowly poured into 28 g of crushed ice, which immediately formed a brown precipitate. The mixture was further cooled in an ice bath and the pH was adjusted dropwise to 7 by adding concentrated ammonium hydroxide solution.Subsequently, the reaction slurry was allowed to stand at -10 °C overnight. On the following day, the precipitate was collected by filtration through a Büchner funnel, washed thoroughly with pre-cooled deionized water and finally dried in a vacuum drying oven to afford the target product 4-amino-2,6-dichloro-3-nitropyridine (1.30 g, 78% yield) as a light tan solid.
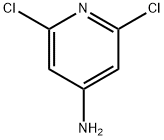
2587-02-2
314 suppliers
$12.40/250mg

2897-43-0
177 suppliers
$7.00/250mg
Yield:2897-43-0 94%
Reaction Conditions:
Stage #1:2,6-dichloro-[4]pyridylamine with sulfuric acid at -5;
Stage #2: with nitric acid in water at 0 - 80;
Stage #3: with ammonia in water; pH=~ 4Cooling with acetone-dry ice;
Steps:
2.G1
Procedure Gl : 2,6-Dichloro-3-nitropyridin-4-amine (X).; To 40 mL of concentrated H2SO4 in a round bottom flask at -5 °C (NaCl/ice bath) was added 3.0 g (18.5 mmol, 1.0 eq.) of 2,6-dichloropyridin-4-amine. The mixture was stirred at -5 °C until a homogenous solution was obtained. A solution of 2.0 g (22.2 mmol, 1.2 eq.) of 70% HNO3 in 2 mL of concentrated H2SO4 was slowly added such that the internal reaction temperature was kept below 10 °C. The mixture was stirred at 0 - 10 °C for 30 min. The mixture was allowed to warm to room temperature and stirred for 30 min. The mixture was then heated to 80 °C and stirred for an additional 1 hr. The mixture was allowed to cool to room temperature and poured onto 200 g of ice with stirring. The yellow suspension was neutralized by the slow addition of concentrated aqueous NH3 to pH~4 while cooling with a dry ice/acetone bath. The yellow solid was collected by filtration, washed with ice cold water and dried under high vacuum to provide 3.6 g (94%) of 2,6-dichloro-3-nitropyridin-4- amine (X) as a yellow solid; 1H-NMR (δH, 300 MHz, CDCl3), 5.77 (bs, 2H), 6.71 (s, IH).
References:
LIGAND PHARMACEUTICALS INC.;COLE, Andrew;MCGUINNESS, Brian, F.;SHAO, Yuefei;DONG, Guizhen;HENDERSON, Ian WO2010/8775, 2010, A1 Location in patent:Page/Page column 24

2587-03-3
22 suppliers
inquiry

2897-43-0
177 suppliers
$7.00/250mg
2402-78-0
513 suppliers
$6.00/25g

2897-43-0
177 suppliers
$7.00/250mg
2587-00-0
193 suppliers
$20.00/1g

2897-43-0
177 suppliers
$7.00/250mg
25194-01-8
184 suppliers
$10.00/1g

2897-43-0
177 suppliers
$7.00/250mg