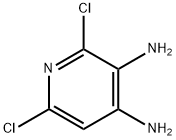
2,6-DICHLOROPYRIDINE-3,4-DIAMINE synthesis
- Product Name:2,6-DICHLOROPYRIDINE-3,4-DIAMINE
- CAS Number:101079-63-4
- Molecular formula:C5H5Cl2N3
- Molecular Weight:178.02
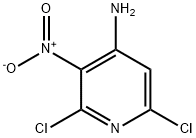
2897-43-0

101079-63-4
The general procedure for the synthesis of 2,6-dichloro-3,4-pyridinediamine from 4-amino-2,6-dichloro-3-nitropyridine was as follows: 2,6-dichloro-3-nitro-4-pyridinamine (881 mg, 4.24 mmol) was dissolved in ethanol (15 mL), followed by the addition of stannic chloride(II) (3212 mg, 16.94 mmol) in batches over a period of 5 minutes . The reaction mixture was stirred under nitrogen protection at 50°C for 3 h. Monitoring by LCMS showed about 60% conversion. After continuing the reaction for 3 h, LCMS analysis showed that the conversion was almost complete. The reaction solution was cooled to room temperature and extracted by partitioning with saturated aqueous sodium bicarbonate (50 mL) and ethyl acetate (50 mL). The organic layer was dried over hydrophobic glass material, concentrated and dried under vacuum overnight to afford the target compound 2,6-dichloro-3,4-pyridinediamine as a yellow solid (734 mg).LCMS (Method B) analysis showed retention time (Rt) = 0.57 min and molecular ion peak (MH+) = 178.
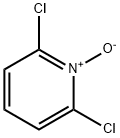
2587-00-0
193 suppliers
$20.00/1g

101079-63-4
125 suppliers
$25.00/100mg
Yield:-
Steps:
Multi-step reaction with 6 steps
1: HNO3; H2SO4 / 160 °C
2: AcOH; Fe / Heating
3: HNO3; H2SO4 / 20 °C
4: H2SO4 / 100 °C
5: 51 percent / Fe / 2 h / Heating
6: aq. NaOH / Heating
References:
Seley, Katherine L.;O'Daniel, Peter I.;Salima, Samer [Nucleosides, nucleotides and nucleic acids,2003,vol. 22,# 12,p. 2133 - 2144]

2897-43-0
177 suppliers
$7.00/250mg

101079-63-4
125 suppliers
$25.00/100mg
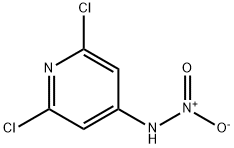
2587-03-3
22 suppliers
inquiry

101079-63-4
125 suppliers
$25.00/100mg
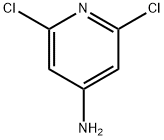
2587-02-2
313 suppliers
$12.40/250mg

101079-63-4
125 suppliers
$25.00/100mg
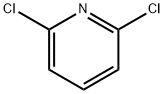
2402-78-0
513 suppliers
$6.00/25g

101079-63-4
125 suppliers
$25.00/100mg