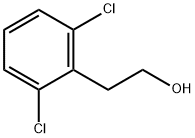
2,6-DICHLOROPHENETHYLALCOHOL synthesis
- Product Name:2,6-DICHLOROPHENETHYLALCOHOL
- CAS Number:30595-79-0
- Molecular formula:C8H8Cl2O
- Molecular Weight:191.05
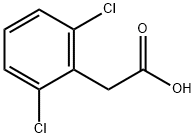
6575-24-2

30595-79-0
(vii) Synthesis of 2,6-dichlorophenylethanol: A suspension of lithium aluminum hydride (13.75 g, 365.75 mmol) was slowly added to a solution of anhydrous ether (500 mL) containing 2,6-dichlorophenylacetic acid (50 g, 243.75 mmol) through a powder charging funnel under nitrogen protection. The reaction mixture was heated to reflux and maintained for 16 hours. Upon completion of the reaction, the reaction was quenched by slow dropwise addition of a saturated aqueous solution of sodium sulfate (25 mL). The resulting slurry was stirred at room temperature for 3 hours, followed by filtration through a Brinell funnel and washing the solid residue with ether (2 x 100 mL). The ether layer filtrates were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent to give 38.6 g (85% yield) of 2,6-dichlorophenethyl alcohol as a colorless oil.
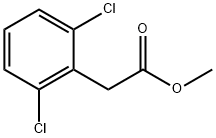
54551-83-6
112 suppliers
$30.00/1g

30595-79-0
143 suppliers
$8.00/250mg
Yield:-
Reaction Conditions:
with sodium tetrahydroborate in tetrahydrofuran;water at 5 - 10; for 4 h;
Steps:
1
2,6-Dichloro phenyl acetic acid methyl ester (1 eq) was taken in THF: Water (10:0.1(v/v) and cooled to 5-10 0C. NaBH4 was added to this mixture and mixture was stirred for 4 h. Mixture was quenched in water and extracted with EtOAc. Organic layer was washed with brine and evaporated in vacuo to afford title compound as thick liquid.
References:
CADILA HEALTHCARE LIMITED WO2009/87649, 2009, A1 Location in patent:Page/Page column 23

6575-24-2
297 suppliers
$8.00/5g

30595-79-0
143 suppliers
$8.00/250mg