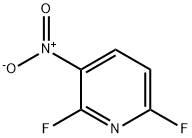
2,6-Difluoro-3-nitropyridine synthesis
- Product Name:2,6-Difluoro-3-nitropyridine
- CAS Number:58602-02-1
- Molecular formula:C5H2F2N2O2
- Molecular Weight:160.08
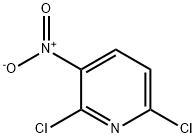
16013-85-7

58602-02-1
The general procedure for the synthesis of 2,6-difluoro-3-nitropyridine from 2,6-dichloro-3-nitropyridine was as follows: 3-bromo-2,6-dichloropyridine (4.70 g, 20.7 mmol) was dissolved in DMSO (103 mL) at room temperature, followed by the addition of cesium fluoride (12.6 g, 82.9 mmol). The reaction mixture was stirred at 80°C for 8 hours in air. After completion of the reaction, the mixture was poured into water and extracted with ether (Et2O). The organic layer was separated, washed sequentially with water and brine, dried over anhydrous sodium sulfate (Na2SO4) and subsequently concentrated under reduced pressure (400 Torr, 40°C). The residue was purified by silica gel column chromatography using ethyl acetate (EtOAc) solution in hexane as eluent to afford 3-bromo-2,6-difluoropyridine (3B) as a colorless oil (2.58 g, 64% yield). Its nuclear magnetic resonance hydrogen spectrum (1H NMR, CDCl3) data were as follows: δ 6.79 (1H, dd, J = 8.3, 3.0 Hz), 8.03 (1H, ddd, J = 8.4, 8.4, 7.0 Hz). Nuclear Magnetic Resonance Fluorine Spectroscopy (19F NMR, CDCl3) data: δ -69.3 Hz, -63.8 Hz. compounds 4B to 8B were prepared similarly as described for 3B.
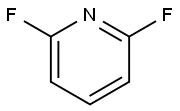
1513-65-1
344 suppliers
$6.00/5g
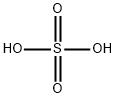
7664-93-9
0 suppliers
$21.64/1003

58602-02-1
78 suppliers
$12.00/250mg
Yield: 74%
Reaction Conditions:
with nitric acid
Steps:
IV.a a
a 2,6-Difluoro-3-nitropyridine To a mixture of concentrated sulphuric acid (600 mL) and fuming nitric acid (90%, 400 mL) in a ice-bath (internal temperature maintained at 5-10° C.) was added drop-wise 2,6-difluoropyridine (200 g, 1.74 mol). The resulting mixture was stirred overnight at room temperature. The mixture was poured slowly into 3 kg of ice and extracted with Et2O (2*2 L). The combined organic layers were washed with aqueous 1.5 N NaOH (2*1 L), then with aqueous saturated NaHCO3 (400 mL) or until pH is around 8-9. The organic layers were dried over MgSO4, filtrated and concentrated under reduced pressure until constant weight (to remove unreacted 2,6-difluoropyridine: 10-12%). The title compound was obtained as a yellow liquid (207.3 g, 74% yield).
References:
Simoneau, Bruno US2002/28807, 2002, A1

16013-85-7
497 suppliers
$9.00/5g

58602-02-1
78 suppliers
$12.00/250mg

1513-65-1
344 suppliers
$6.00/5g

58602-02-1
78 suppliers
$12.00/250mg