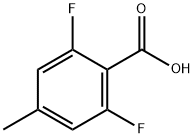
2,6-difluoro-4-methylbenzoic acid synthesis
- Product Name:2,6-difluoro-4-methylbenzoic acid
- CAS Number:1201597-23-0
- Molecular formula:C8H6F2O2
- Molecular Weight:172.13
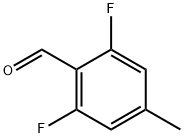
1201597-22-9

1201597-23-0
Step C: Synthesis of 2,6-difluoro-4-methylbenzoic acid 1. silver oxide (43.8 g, 0.189 mol), water (200 mL) and sodium hydroxide (33.7 g, 0.842 mol) were added to a flask. 2. 2,6-difluoro-4-methylbenzaldehyde (29.23 g, 0.187 mol) was added in batches over 30 min. 3. intense exothermic phenomena were observed during the reaction and the color of the reaction mixture changed from black to gray. 4. The thick suspension formed was continued to be stirred for 1 hour and subsequently filtered through a Brinell funnel. 5. The filtrate was acidified to pH 2 with concentrated hydrochloric acid to produce a suspension. 6. The precipitate was collected by filtration, dissolved in ether and dried by adding anhydrous sodium sulfate. 7. The dried solution was filtered and concentrated to give a white solid product, 2,6-difluoro-4-methylbenzoic acid (17.0 g, 53% yield). 8. The product was purified by 1H-NHPA and concentrated. 8. The product was characterized by 1H-NMR (500 MHz, d6-DMSO): δ 13.7 (br.s, 1H), 7.02 (d, 2H, J = 9.3 Hz), 2.32 (s, 3H).

1201597-22-9
50 suppliers
$215.00/100mg

1201597-23-0
60 suppliers
$36.00/100mg
Yield: 53%
Reaction Conditions:
Stage #1:2,6-difluoro-4-methylbenzaldehyde with sodium hydroxide;silver(l) oxide in water for 1.5 h;
Stage #2: with hydrogenchloride in water; pH=2
Steps:
C
Silver oxide (43.8 g, 0.189mol) was placed in a flask along with water (20OmL) and sodium hydroxide (33.7g, 0.842mol). To it was added 2,6-difluoro-4-methylbenzaldehyde (29.23 g, 0.187mol) in small portions over a 30min period. After a vigorous exothermic reaction, the color of the reaction mixture changed from black to gray. The resulting thick suspension was stirred for 1 hr and then filtered through a Buchner funnel. The filtrate was acidified to pH 2 with concentrated HCl to give a suspension. The precipitate was collected by suction filtration, dissolved in ether, dried over anhydrous Na2SO4, filtered and concentrated to give white solid (17.0 g, 53%). 1H-NMR (500MHz, d6-DMSO): 613.7 ( br.s, 1H ), 7.02 ( d, 2H, J=9.3 Hz), 2.32 ( s, 3H ).
References:
MERCK SHARP &;DOHME CORP.;KOYAMA, Hiroo;SAHOO, Soumya, P.;YANG, Ginger Xu-Qiang;MILLER, Daniel, J. WO2010/129208, 2010, A1 Location in patent:Page/Page column 32-33

124-38-9
134 suppliers
$175.00/23402
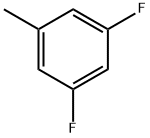
117358-51-7
153 suppliers
$19.00/1g

1201597-23-0
60 suppliers
$36.00/100mg

117358-51-7
153 suppliers
$19.00/1g

1201597-23-0
60 suppliers
$36.00/100mg
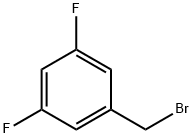
141776-91-2
253 suppliers
$5.00/1g

1201597-23-0
60 suppliers
$36.00/100mg