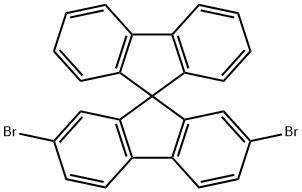
2,7-Dibromo-9,9'-spiro-bifluorene synthesis
- Product Name:2,7-Dibromo-9,9'-spiro-bifluorene
- CAS Number:171408-84-7
- Molecular formula:C25H14Br2
- Molecular Weight:474.19
![9-([1,1'-Biphenyl]-2-yl)-2,7-dibromo-9H-fluoren-9-ol](/CAS/20210305/GIF/171408-85-8.gif)
171408-85-8

171408-84-7
General procedure for the synthesis of 2,7-dibromo-9,9'-spirobifluorene from the compound (CAS: 171408-85-8): to a toluene solution containing 180 g of 9-(2-biphenylyl)-2,7-dibromo-9-fluorenol (the product of Example 3), the reaction was initiated by the slow addition of 18.0 g (0.180 mol, 2.00 M) of concentrated sulfuric acid. The reaction mixture was stirred at room temperature for 2 hours. Upon completion of the reaction, aqueous caustic soda solution was added dropwise to the mixture until neutral, followed by the addition of toluene to promote crystallization. The precipitated white crystals were collected by filtration and washed with an appropriate amount of toluene and dried to afford 35.9 g of 2,7-dibromo-9,9'-spirobifluorene (yield: 80%, based on Example 3 the total yield was 72%, HPLC purity ≥95%).
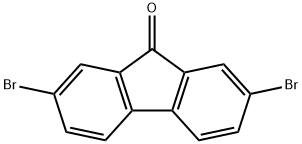
14348-75-5
312 suppliers
$14.00/1g
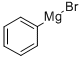
100-58-3
196 suppliers
$18.00/10ml
583-53-9
322 suppliers
$10.00/5g

171408-84-7
215 suppliers
$8.00/1g
Yield:171408-84-7 76.2%
Reaction Conditions:
Stage #1:phenylmagnesium bromide;2,3-dibromobenzene in 2-methyltetrahydrofuran at 80; for 12 h;Inert atmosphere;
Stage #2:2,7-dibromofluorene-9-one in 2-methyltetrahydrofuran;diethyl ether for 2 h;Reflux;
Stage #3: with hydrogenchloride;acetic acid in 2-methyltetrahydrofuran;diethyl ether at 75; for 4 h;
Steps:
2
A solution of 10 mL of o-dibromobenzene (0.1 mmol) in methyl tetrahydrofuran was added dropwise to a solution of 5 mL of phenylmagnesium bromide (0.11 mmol) in methyltetrahydrofuran under argon atmosphere at 80 ° C. Reaction 12 h. The reaction solution was then added dropwise to 10 mL of 2, 7-dibromofluorenone (0.1 mmol) in ether. The mixture was heated to reflux for 2 h, hydrolyzed and filtered. The solid was dissolved in 5 mL of mixed acid (glacial acetic acid and hydrochloric acid) (200-300 mesh silica gel) to give the product 2, 7-dibromo-9, 9 '-spirobifluorene , which was purified by column chromatography on silica gel eluted with a mixed solvent of methylene chloride and n-hexane.36 . 2 mg, yield 76.2%. The resulting 2, 7-dibromo-9,9-spirobifluorene was reacted with lithium diphenylphosphine (0.15 mmol) in tetrahydrofuran solvent. After refluxing for 12 h, methanol was added to the reaction solution, (200-300 mesh silica gel) to obtain the desired product. 2-Bis (diphenylphosphino) -9, 9-spirobifluorene 35. 9 mg was obtained by the same procedure as that of Example 1, Mg, and the yield was 70.1%.
References:
Henan Institute of Chemical Sciences Co.,Ltd;YANG, ZHENQIANG;YANG, RUINA;JIANG, WEIPENG;ZHOU, DUO;FENG, PEIPEI;QU, FENGBO CN103333204, 2016, B Location in patent:Paragraph 0023; 0024; 0025
![9-([1,1'-Biphenyl]-2-yl)-2,7-dibromo-9H-fluoren-9-ol](/CAS/20210305/GIF/171408-85-8.gif)
171408-85-8
1 suppliers
inquiry

171408-84-7
215 suppliers
$8.00/1g
2052-07-5
397 suppliers
$16.30/1g

14348-75-5
312 suppliers
$14.00/1g

171408-84-7
215 suppliers
$8.00/1g
![9,9'-Spirobi[9H-fluorene]](/CAS/GIF/159-66-0.gif)
159-66-0
220 suppliers
$9.00/1g

171408-84-7
215 suppliers
$8.00/1g

14348-75-5
312 suppliers
$14.00/1g

171408-84-7
215 suppliers
$8.00/1g