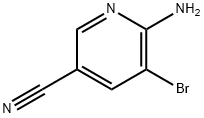
2-Amino-3-bromo-5-cyanopyridine synthesis
- Product Name:2-Amino-3-bromo-5-cyanopyridine
- CAS Number:477871-32-2
- Molecular formula:C6H4BrN3
- Molecular Weight:198.02
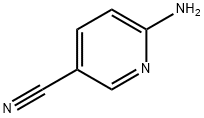
4214-73-7

477871-32-2
The general procedure for the synthesis of 6-amino-5-bromonicotinonitrile from 2-amino-5-cyanopyridine is as follows: Step 1: To a solution of 6-aminonicotinonitrile (102 mg, 0.86 mmol) in acetic acid (2 mL) was added a solution of bromine in acetic acid (0.86 mmol). The mixture was stirred at room temperature for 2 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure. The crude product was purified by rapid chromatography on silica gel (eluent: hexane/ethyl acetate, 3:1, v/v) to afford the title compound 6-amino-5-bromonicotinonitrile (110 mg, 65% yield). The product characterization data are as follows: 1H-NMR (DMSO, 300 MHz): δ 8.26 (d, 1H, J = 2.0 Hz), 8.10 (d, 1H, J = 2.0 Hz), 7.24 (bs, 2H). MS (APCI Neg): m/z 196, 198.

4214-73-7
304 suppliers
$5.00/1g

477871-32-2
94 suppliers
inquiry
Yield: 95%
Reaction Conditions:
with N-Bromosuccinimide in acetonitrile at 20;
Steps:
4.1 1) Synthesis of Compound 4b
A reaction was performed at room temperature while stirring 2.00 g (16.8 mmol, 1 equivalent) of Compound 4a and 1.2 equivalents of N-bromosuccinimide under an acetonitrile solvent. After the reaction was terminated, water was poured thereto. Extraction was performed by using chloroform, the extract was washed with Na2S2O3 (aq), and then the moisture was removed over anhydrous magnesium sulfate. 3.16 g (yield 95%) of Compound 4b could be obtained by concentrating the residue through distillation under reduced pressure.
References:
LG CHEM, LTD.;LEE, Milim;SON, Seonkyoung;SONG, Cheol Jun;MOON, Sang Pil;LEE, Hoyong US2020/95265, 2020, A1 Location in patent:Paragraph 0140-0141