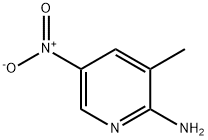
2-Amino-3-methyl-5-nitropyridine synthesis
- Product Name:2-Amino-3-methyl-5-nitropyridine
- CAS Number:18344-51-9
- Molecular formula:C6H7N3O2
- Molecular Weight:153.14
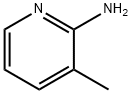
1603-40-3

18344-51-9
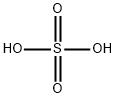
The general procedure for the synthesis of 2-amino-3-methyl-5-nitropyridine from 2-amino-3-methylpyridine was as follows: 3-methylpyridin-2-amine (5.0 g, 46.2 mmol) was dissolved in concentrated sulfuric acid (24 mL) and the mixture was cooled to 0 °C. At the same time, fuming nitric acid (density = 1.5, 3.5 mL) was cooled to 0 °C. Concentrated sulfuric acid (3.5 mL) was slowly added dropwise to the reaction mixture while keeping the temperature below 20°C. Subsequently, the stirred mixture was gradually warmed to 20 °C and transferred to a second flask in 3-5 mL batches. The second flask was heated to 35-40°C (note: the temperature should not exceed 40°C and the temperature should be carefully monitored after each addition of a new batch). Next, the reaction mixture was continued to be stirred at 50°C for 30 minutes. Once the reaction is complete, the mixture is cooled to room temperature and neutralized with concentrated ammonia. At this point, a precipitate is generated, which is filtered and washed sequentially with water and 50% aqueous DMFA (6 mL). Finally, the product was purified by recrystallization from DMFA to give 3-methyl-5-nitropyridin-2-amine (2.52 g, 35% yield).

1603-40-3
488 suppliers
$6.00/25g

18344-51-9
241 suppliers
$8.00/5g
Yield: 35%
Reaction Conditions:
Stage #1:3-methylpyridin-2-ylamine with sulfuric acid;nitric acid at 0 - 50;
Stage #2: with ammonia in water at 20;
Steps:
19.1
3-Methyl-pyridin-2-ylamine (5.0 g, 46.2 mmol) was dissolved in concentrated sulfuric acid (24ml), the mixture was chilled to 0°C and a mixture of fuming nitric acid (d=1.5, 3.5ml) and concentrated sulfuric acid (3.5ml) was added dropwise to the reaction mixture while temperature was kept below 20°C. The stirred mixture was allowed to warm to 20°C and transferred in portions of 3-5ml into a second flask which was heated to 35-40°C (the temperature was not allowed to rise over 40°C - monitoring carefully the temperature after every addition of a new portion). The resulting reaction mixture was subsequently stirred for additional 30min at 50°C, cooled to ambient temperature and neutralized with concentrated aqueous ammonia. This led to the formation of a precipitated which was filtered, washed with water and aqueous DMFA (50%, 6ml), and recrystallized from DMFA yielding 3-methyl-5-nitro-pyridin-2-ylamine (2.52g, 35%).
References:
Location in patent:Page/Page column 36
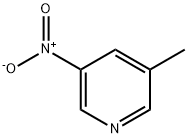
6960-20-9
81 suppliers
$60.00/500mg

18344-51-9
241 suppliers
$8.00/5g
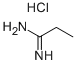
3599-89-1
96 suppliers
$15.00/250mg
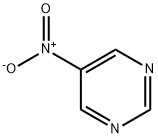
14080-32-1
32 suppliers
inquiry

18344-51-9
241 suppliers
$8.00/5g

79899-27-7
3 suppliers
inquiry
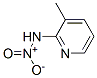
18344-53-1
1 suppliers
inquiry

18344-51-9
241 suppliers
$8.00/5g