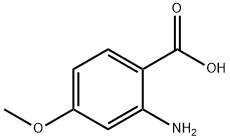
2-AMINO-4-METHOXY-BENZOIC ACID synthesis
- Product Name:2-AMINO-4-METHOXY-BENZOIC ACID
- CAS Number:4294-95-5
- Molecular formula:C8H9NO3
- Molecular Weight:167.16
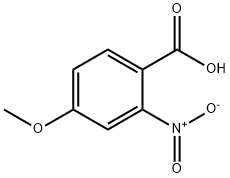
33844-21-2

4294-95-5
The general procedure for the synthesis of 2-amino-4-methoxybenzoic acid from 4-methoxy-2-nitrobenzoic acid is as follows: Intermediate 113: Synthesis of 2-amino-4-methoxybenzoic acid 4-Methoxy-2-nitrobenzoic acid (3 g, 16.4 mmol) was dissolved in methanol (80 mL) and 10% palladium carbon catalyst (300 mg) was added. The hydrogenation reaction was carried out at room temperature and atmospheric pressure for 18 hours. Upon completion of the reaction, the reaction mixture was filtered through diatomaceous earth to remove the catalyst and the filtrate was concentrated to dryness under reduced pressure to give 2.50 g (100% yield) of 2-amino-4-methoxybenzoic acid as a colorless solid. 1H NMR (DMSO-d6) δ: 3.70 (s, 3H); 6.09 (dd, 1H); 6.23 (d, 1H); 7.59 (d, 1H).

33844-21-2
229 suppliers
$15.00/1 g

4294-95-5
246 suppliers
$14.00/1g
Yield:4294-95-5 100%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol at 20; under 760.051 Torr; for 18 h;
Steps:
113
Intermediate 113: 2-Amino-4-methoxybenzoic acid4-Methoxy-2-nitrobenzoic acid (3 g, 16.4 mmol) was hydrogenated over palladium on carbon (10%, 300 mg) in methanol (80 mL) at room temperature and normal pressure for 18 hours. The reaction mixture was filtered through celite and concentrated to dryness under reduce pressure to give 2.50 g (100%) of the product as a colorless solid.1H NMR (DMSO-d^ δ 3.70 (s, 3H); 6.09 (dd, IH); 6.23 (d, IH); 7.59 (d, IH).
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED WO2006/134378, 2006, A1 Location in patent:Page/Page column 112
38487-91-1
42 suppliers
$146.66/0.5g

4294-95-5
246 suppliers
$14.00/1g
50413-30-4
143 suppliers
$16.00/250mg

4294-95-5
246 suppliers
$14.00/1g
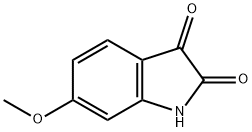
52351-75-4
140 suppliers
$7.00/100mg

4294-95-5
246 suppliers
$14.00/1g

33844-21-2
229 suppliers
$15.00/1 g
7440-05-3
508 suppliers
$17.69/1G

4294-95-5
246 suppliers
$14.00/1g