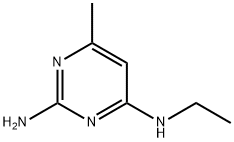
2-amino-4-methyl-6-ethylaminopyrimidine synthesis
- Product Name:2-amino-4-methyl-6-ethylaminopyrimidine
- CAS Number:934493-92-2
- Molecular formula:C7H12N4
- Molecular Weight:152.2
5600-21-5
322 suppliers
$18.00/25g
75-04-7
405 suppliers
$10.00/20mg

934493-92-2
4 suppliers
inquiry
Yield:934493-92-2 100%
Reaction Conditions:
with triethylamine in water; for 20 h;Heating / reflux;
Steps:
3
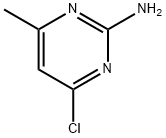
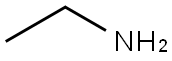
Intermediate 3; 2-Amino-6-bromo-8-ethyl-4-methylpyrido[2,3-d]pyrimidin-7(8JH)-one; [00219] To a 3-necked 3-L flask, that was equipped with an overhead stirrer, was added in order 2-amino~4-chloro-6-methylpyrimidine (Aldrich, 100 g, 0.696 mol, 1 equiv.), ethylamine (70% ethylamine in water, Lancaster, 625 mL), 625 niL H2O, and 125 niL TEA (0.889 mol, 1.28 equiv.). The mixture was stirred and heated at reflux for 20 h, during which time the reaction turned homogeneous. The reaction was allowed to cool to room temperature. The volatile ethylamine was removed on a rotary evaporator. A precipitate formed. The aqueous mixture containing the precipitate was allowed to stand at room temperature for 2 h and then filtered. After drying under vacuum, 106 g (100% yield) of 2-amino-6-ethylaminopyrimidine was obtained as a colorless solid. This material was used as such in the following reaction.
References:
WO2007/44698,2007,A1 Location in patent:Page/Page column 69
