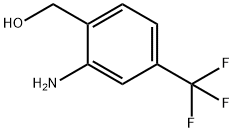
2-Amino-4-(trifluoromethyl)benzenemethanol synthesis
- Product Name:2-Amino-4-(trifluoromethyl)benzenemethanol
- CAS Number:186602-93-7
- Molecular formula:C8H8F3NO
- Molecular Weight:191.15
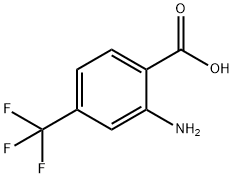
402-13-1

186602-93-7
General procedure for the synthesis of (2-amino-4-(trifluoromethyl)phenyl)methanol from 2-amino-4-trifluoromethylbenzoic acid: representative method for the preparation of 2-amino-4-(trifluoromethyl)benzyl alcohol (17a): 2-amino-4-trifluoromethylbenzoic acid (16a, 2.04 g, 10 mmol) and NaBH4 (0.912 g, 24 mmol) were were suspended in THF (10 mL). A solution of iodine (I2, 2.54 g, 10 mmol) in THF (10 mL) was slowly added at 0°C over 10-15 min under nitrogen protection. The reaction mixture was stirred at room temperature for 30 min, followed by heating and reflux stirring for 24 hr. Upon completion of the reaction, the mixture was cooled to room temperature and MeOH was slowly added until a clarified solution was formed. The solvent was removed under reduced pressure and the residue was stirred with 20% aqueous KOH solution (20 mL) for 4 hours at room temperature. The reaction mixture was extracted with CH2Cl2 (3 x 100 mL) and the organic phase was dried with anhydrous Na2SO4 and concentrated under reduced pressure to give the target alcohol 17a (1.91 g, 100% yield).1H NMR (CDCl3, 300 MHz): δ 7.15 (d, 1H, J = 7.5 Hz), 6.94 (d, 1H, J = 7.5 Hz), 6.91 ( s, 1H), 4.71 (s, 2H).

402-13-1
171 suppliers
$18.00/5g

186602-93-7
27 suppliers
inquiry
Yield:186602-93-7 100%
Reaction Conditions:
with sodium tetrahydroborate;iodine in tetrahydrofuran; for 24 h;Heating / reflux;
Steps:
[0069] Representative procedure for the preparation of 2-amino-4- (trifluoromethyl) benzylalcohol (17a): To a suspension of ANTHRANILIC acid 16a (2. 04 g, 10 MMOL), NaBH4 (0. 912 g, 24 MMOL) in THF (10 mL) under N2 at 0 °C was added 12 (2.54 g, 10 MMOL) solution in THF (10 mL) over a period of 10-15 minutes. The resulting reaction mixture was stirred at room temperature for 30 minutes and then at reflux for 24 h. The reaction mixture was cooled to room temperature and MeOH was added very slowly to the mixture till the clear solution was formed. The solvent was removed and the residue was stirred with 20% KOH (20 mL) at room temperature for 4 h, extracted with CH2CI2 (3 x 100 mL), dried (NA2SO4) and solvent was removed to obtain 1.91 g (100%) of the requisite ALCOHOL 17A. 1H NMR (CDC13, 300 MHz): 8 7.15 (d, 1H, J = 7.5 Hz), 6.94 (d, 1H, J = 7.5 Hz), 6.91 (s, 1H), 4.71 (s, 2H).
References:
WO2005/30774,2005,A1 Location in patent:Page/Page column 20
133605-27-3
46 suppliers
$71.79/1g

186602-93-7
27 suppliers
inquiry
320-94-5
233 suppliers
$9.00/1g

186602-93-7
27 suppliers
inquiry
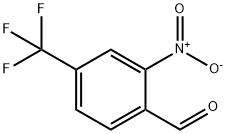
109466-87-7
86 suppliers
$20.00/250mg

186602-93-7
27 suppliers
inquiry
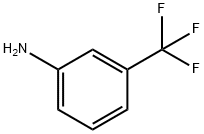
98-16-8
391 suppliers
$9.00/5g

186602-93-7
27 suppliers
inquiry