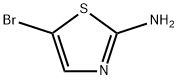
2-Amino-5-bromothiazole synthesis
- Product Name:2-Amino-5-bromothiazole
- CAS Number:3034-22-8
- Molecular formula:C3H3BrN2S
- Molecular Weight:179.04
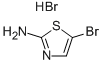
61296-22-8

3034-22-8
General procedure for the synthesis of 2-amino-5-bromothiazole from 2-amino-5-bromothiazole hydrobromide: A suspension of 2-amino-5-bromothiazole hydrobromide (30.0 g, 116.35 mmol) and triethylamine (TEA, 24.1 mL, 174.53 mmol) in tetrahydrofuran (THF, 350 mL) was stirred for 6 hours at room temperature. Upon completion of the reaction, the resulting precipitate was removed by filtration and the filtrate was concentrated under reduced pressure to afford 2-amino-5-bromothiazole (17 g, 94.97 mmol). The resulting product could be used directly in the subsequent reaction without further purification.
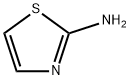
96-50-4
555 suppliers
$5.00/5g

3034-22-8
206 suppliers
$7.00/250mg
Yield:3034-22-8 75%
Reaction Conditions:
with bromine;acetic acid at 0 - 30; for 2 h;
Steps:
5.a
(a)at 0 ° C2-Aminothiazole (400 mg, 4 mmol) was dissolved in 16 mLAcetic acid solution,Bromine (408 μL, 8 mmol) was slowly added dropwise.The mixture was further stirred at room temperature for 2 hours,TLC reaction was monitored until the reaction was completed,The pH was adjusted to 7-8 with saturated NaHCO3,Ethyl acetate (20 mL x 3), washed with saturated saline and the organic layer was mixed,Dry over anhydrous sodium sulfate, filter and concentrate and purify by column chromatography5-Bromothiazol-2-amine(520 mg, 75% yield).
References:
Chinese Academy Of Sciences Shanghai Organic Chemistry Institute;Zhu Jidong;Gu Shoulai;Si Xiaojia;Xie Jingjing;Shen Jian CN107286150, 2017, A Location in patent:Paragraph 0354; 0355; 0356

61296-22-8
265 suppliers
$6.00/1g

3034-22-8
206 suppliers
$7.00/250mg
7336-54-1
87 suppliers
$45.00/250 mg

3034-22-8
206 suppliers
$7.00/250mg
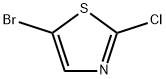
3034-56-8
73 suppliers
$32.00/250mg

3034-22-8
206 suppliers
$7.00/250mg
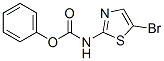
226879-69-2
2 suppliers
inquiry

3034-22-8
206 suppliers
$7.00/250mg

108-95-2
786 suppliers
$14.00/25g