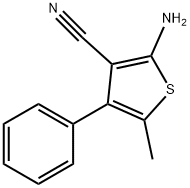
2-AMINO-5-METHYL-4-PHENYLTHIOPHENE-3-CARBONITRILE synthesis
- Product Name:2-AMINO-5-METHYL-4-PHENYLTHIOPHENE-3-CARBONITRILE
- CAS Number:99011-93-5
- Molecular formula:C12H10N2S
- Molecular Weight:214.29
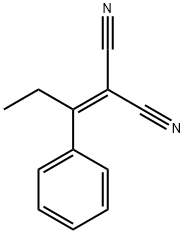
10432-39-0
3 suppliers
inquiry

99011-93-5
14 suppliers
inquiry
Yield:99011-93-5 81%
Reaction Conditions:
with sulfur;triethylamine in ethanol at 60; for 12 h;
Steps:
9 Second step
A 2OmL vial is charged with 2-(1-Phenyl- propylidene)malononitrile (5 mmol), sulfur (5 mmol), and triethylamine (5 mmol) in ethanol (5 mL, 1.0 M solution) . The reaction is heated 60 ° C for 12 h. Then, the reaction was cooled down to room temperature. Evaporation of ethanol left a residue that was purified by column chromatography Yield: 81%.1H NMR (500 MHz, CDC13) 6 7.46-7.40 (m, 2H), 7.38-7.33 (m, 3H), 4.70 (br, 2H), 2.24 (s, 3H) . 13C NMR (126 MHz, CDC13) 6159.9, 135.1, 133.7, 129.1, 128.5, 127.8, 119.2, 115.9, 89.8,13.4. MS (El) m/z 215 (M+)
References:
WO2017/118762,2017,A1 Location in patent:Page/Page column 37
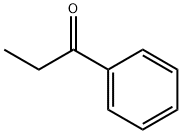
93-55-0
7 suppliers
$15.00/25g

99011-93-5
14 suppliers
inquiry