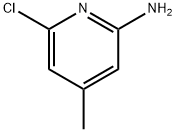
2-Amino-6-chloro-4-picoline synthesis
- Product Name:2-Amino-6-chloro-4-picoline
- CAS Number:51564-92-2
- Molecular formula:C6H7ClN2
- Molecular Weight:142.59
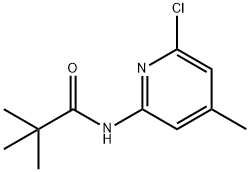
1004294-60-3

51564-92-2
Step d: Synthesis of 6-chloro-4-methylpyridin-2-amine; N-(6-chloro-4-methylpyridin-2-yl) neopentanamide (500 mg, 2.21 mmol) was dissolved in 6M hydrochloric acid solution (40 mL) and the reaction was stirred at 90 °C for 6 h. The reaction was carried out at 90 °C for 2 h. The reaction was carried out at 90 °C for 1 h. The reaction was carried out at 90 °C for 2 h. The reaction was carried out at 90 °C for 2 h. The reaction was carried out at 90 °C for 2 h. The reaction was carried out at 90 °C for 2 h. The reaction was carried out at 90 °C for 2 h. Upon completion of the reaction, the mixture was cooled to room temperature and neutralized with sodium hydroxide solution to pH 10. Subsequently, the mixture was extracted with ethyl acetate, the organic phases were combined and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography with the eluent being a mixed solution of petroleum ether and ethyl acetate (95:5, v/v) to afford 6-chloro-4-methylpyridin-2-amine (257 mg, 82% yield). The product was characterized by 1H NMR (CDCl3, 300 MHz): δ 6.52 (s, 1H), 6.26 (s, 1H), 2.23 (s, 3H).

1004294-60-3
1 suppliers
inquiry

51564-92-2
110 suppliers
$75.00/250mg
Yield:51564-92-2 82%
Reaction Conditions:
Stage #1: N-(6-chloro-4-methylpyridin-2-yl)pivalamidewith hydrogenchloride;water at 90; for 6 h;
Stage #2: with sodium hydroxide in water at 20; pH=10;
Steps:
J.d
Step d: 6-Chloro-4-methylpyridin-2-amine; A solution of N-(6-chloro-4-methylpyridin-2-yl)pivalamide (500 mg, 2.21 mmol) in HCl (40 mL, 6 M) was stirred for 6 hours at 90 0C. The mixture was cooled to room temperature and neutralized with NaOH to pH 10. The mixture was extracted with ethyl acetate, evaporated under vacuum, and purified by chromatography on silica gel (5% ethyl acetate in petroleum ether) to afford 6-chloro-4-methylpyridin-2-amine (257 mg, 82%). 1H νMR (CDCl3, 300 MHz) δ 6.52 (s, IH), 6.26 (s, IH), 2.23 (s, 3H).
References:
WO2008/141119,2008,A2 Location in patent:Page/Page column 86-87
39621-00-6
161 suppliers
$6.00/1g

51564-92-2
110 suppliers
$75.00/250mg
695-34-1
537 suppliers
$5.00/10g

51564-92-2
110 suppliers
$75.00/250mg
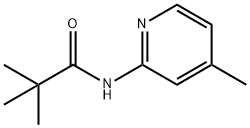
86847-77-0
22 suppliers
inquiry

51564-92-2
110 suppliers
$75.00/250mg

1004294-59-0
0 suppliers
inquiry

51564-92-2
110 suppliers
$75.00/250mg