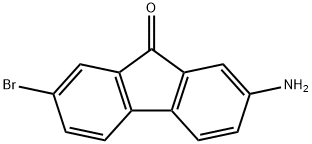
2-AMINO-7-BROMO-9-FLUORENONE synthesis
- Product Name:2-AMINO-7-BROMO-9-FLUORENONE
- CAS Number:58557-63-4
- Molecular formula:C13H8BrNO
- Molecular Weight:274.11
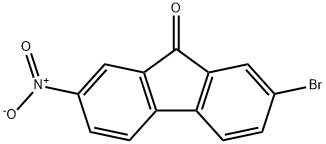
58557-62-3
2 suppliers
inquiry

58557-63-4
19 suppliers
$50.00/250mg
Yield:58557-63-4 94%
Reaction Conditions:
with sodiumsulfide nonahydrate;sodium hydroxide in ethanol;water at 20 - 110;Reflux;
Steps:
2-Amino-7-bromo-9H-fluoren-9-one10: To a stirred suspension of compound 5b (1.035 g, 3.4 mmol) in ethanol (14 mL) was added a solution of Na2S?9H2O (2.22 g, 9.2 mmol) and sodium hydroxide (0.88 g, 22 mmol) in water (36 mL). The mixture was refluxed for 5 h and stirred overnight at room temperature. The resulting deep-brown mixture was cooled to 0-5 °C and the precipitate was filtered off. The crude product was washed successively with H2O (2 x 6 mL), aqueous NaOH (5%, 2 x 6 mL), H2O (3 x 6 mL), cold EtOH (2 x 4 mL), MTBE (5 mL), and pentane (5 mL) and dried in vacuo (0.875 g, 94% yield). A sample (45 mg) was recrystallized from toluene to give 39 mg of 2-amino-7-bromo-9H-fluoren-9-one, as deep violet needles (Rf = 0.39, heptane/ethyl acetate, 3:2). 1H NMR (400 MHz, DMSO-d6) δ 7.63 (dd, J = 8.0, 1.9 Hz, 1HAr, 6-H), 7.51 (d, J = 1.8 Hz, 1HAr, 8-H), 7.42 (d, J = 8.0 Hz, 1HAr, 4-H), 7.39 (d, J = 8.1 Hz, 1HAr, 5-H), 6.81 (d, J = 2.2 Hz, 1HAr, 1-H), 6.69 (dd, J = 8.0, 2.2 Hz, 1HAr, 3-H), 5.72 (s, 2H, NH 2). 13C NMR (101 MHz, DMSO-d6) δC 192.58 (1C, C=O), 150.72 (1CAr, 2-C), 144.75 (1CAr, 5a-C), 137.33 (1CArH, 6-C), 135.01, 134.64 (2CAr, 4a-C, 9a-C), 129.98 (1CAr, 8a-C), 126.15 (1CArH, 8-C), 122.40 (1CArH, 5-C), 121.01 (1CArH, 4-C), 119.10 (1CAr, 7-C), 118.57 (1CArH, 3-C), 109.37 (1CArH, 1-C).
References:
Teodoro, Rodrigo;Scheunemann, Matthias;Wenzel, Barbara;Peters, Dan;Deuther-Conrad, Winnie;Brust, Peter [Bioorganic and Medicinal Chemistry Letters,2018,vol. 28,# 9,p. 1471 - 1475] Location in patent:supporting information
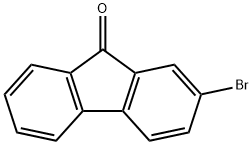
3096-56-8
352 suppliers
$10.00/1g

58557-63-4
19 suppliers
$50.00/250mg
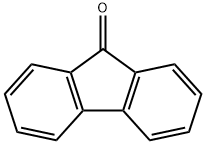
486-25-9
488 suppliers
$5.00/25g

58557-63-4
19 suppliers
$50.00/250mg