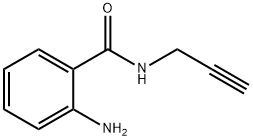
2-AMINO-N-(2-PROPYNYL)BENZENECARBOXAMIDE synthesis
- Product Name:2-AMINO-N-(2-PROPYNYL)BENZENECARBOXAMIDE
- CAS Number:4943-83-3
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
118-48-9
446 suppliers
$5.00/5 g
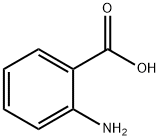
2450-71-7
337 suppliers
$14.00/1g

4943-83-3
25 suppliers
$45.00/50mg
Yield: 100%
Reaction Conditions:
in tetrahydrofuran at 0; for 2 h;
Steps:
2-Amino-N-prop-2-ynyl-benzamide
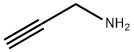
General procedure: Isatoic anhydride (4.00 g, 24.5 mmol) was dissolved in tetrahydrofuran (100 mL) at 0 °C. Prop-2-ynylamine (2.52 mL, 36.8 mmol) was added and the reaction was stirred for 2 hours and then concentrated under reduced pressure. Purification by silica gel chromatography using a gradient of 0-50% Ethyl acetate/hexane as the eluting solvent afforded the desired product as a white solid (4.27 g, 100%). mp 75 °C; LCMS: m/z = 120 (M-NHCH2CCH); 1H NMR (400 MHz, CDCl3) δ 7.35 (d, 1H, J = 7.9 Hz), 7.24 (dd, 1H, J = 7.3 and 8.2 Hz), 6.73-6.64 (m, 2H), 6.19 (br s, 1H), 5.56 (br s, 2H), 4.26-4.20 (m, 2H), 2.29 (t, 1H, J = 1.5 Hz).
References:
Weinberg, Linda R.;Albom, Mark S.;Angeles, Thelma S.;Husten, Jean;Lisko, Joseph G.;McHugh, Robert J.;Milkiewicz, Karen L.;Murthy, Seetha;Ott, Gregory R.;Theroff, Jay P.;Tripathy, Rabindranath;Underiner, Ted L.;Zificsak, Craig A.;Dorsey, Bruce D. [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 1,p. 164 - 167] Location in patent:supporting information; experimental part
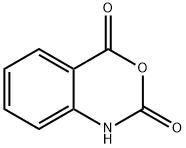
118-92-3
0 suppliers
$21.60/25g

2450-71-7
337 suppliers
$14.00/1g

4943-83-3
25 suppliers
$45.00/50mg
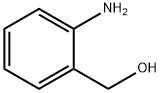
5344-90-1
341 suppliers
$6.00/5g

2450-71-7
337 suppliers
$14.00/1g

4943-83-3
25 suppliers
$45.00/50mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4943-83-3
25 suppliers
$45.00/50mg

118-48-9
446 suppliers
$5.00/5 g

4943-83-3
25 suppliers
$45.00/50mg