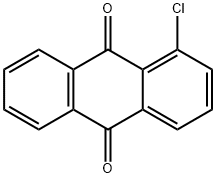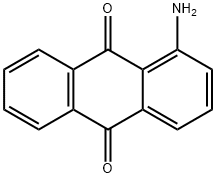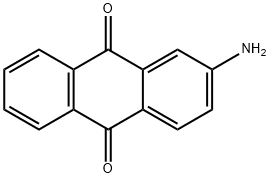
2-AMINOANTHRAQUINONE synthesis
- Product Name:2-AMINOANTHRAQUINONE
- CAS Number:117-79-3
- Molecular formula:C14H9NO2
- Molecular Weight:223.23
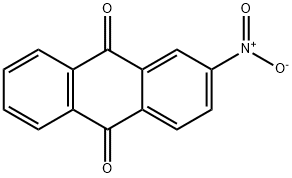
605-27-6
2 suppliers
inquiry

117-79-3
184 suppliers
$14.00/1g
Yield:117-79-3 99%
Reaction Conditions:
with sulfuric acid;palladium-carbon in water;hydrogen
Steps:
6 EXAMPLE 6
EXAMPLE 6 2.5 g (0.01 mole) of 2-nitroanthraquinone (purity above 99%) containing about 10% of particles having a particle diameter of more than 100 microns was placed in a 200 ml. electromagnetically stirred autoclave together with 25 g of small glass balls each with a diameter of 1 mm, 75 g of water and 0.025 g of 5% palladium-carbon. The inside of the autoclave was purged with hydrogen, and then, the 2-nitroanthraquinone was hydrogenated with stirring at a temperature of 100° C. and a pressure of 2 to 6 Kg/cm2.G. The reaction proceeded smoothly, and stopped in 4 hours with the absorption of a theoretical amount of hydrogen. The reaction mixture was filtered, and the 22 g of conc. sulfuric acid was added to the filtrate mass to dissolve the reaction product. Then, the catalyst and the small glass balls were separated by filtration. The filtrate was diluted with water. The precipitated crystals were collected by filtration to afford 2.2 g of 2-aminoanthraquinone. The purity of the product was 99%, and its yield was almost quantitative.
References:
Mitsui Toatsu Chemicals, Inc. US4046785, 1977, A
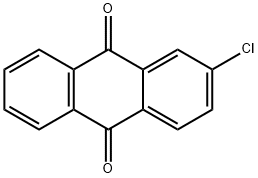
131-09-9
194 suppliers
$10.00/1g

117-79-3
184 suppliers
$14.00/1g
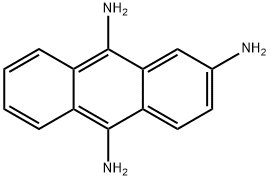
1234379-65-7
0 suppliers
inquiry

117-79-3
184 suppliers
$14.00/1g
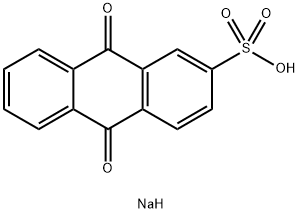
131-08-8
204 suppliers
$5.00/5g

117-79-3
184 suppliers
$14.00/1g