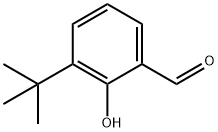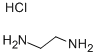
2-Aminoethylammonium chloride synthesis
- Product Name:2-Aminoethylammonium chloride
- CAS Number:18299-54-2
- Molecular formula:C2H9ClN2
- Molecular Weight:96.56
107-15-3
0 suppliers
$11.19/25ML
18299-54-2
80 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride in water; pH=8.5 at -4 - 20; for 1 h;
Steps:
6
5,05 ml (7.5 mmol) of ethylenediamine was dissolved in 75 ml of deionized water, and stirred at room temperature at 300 r/min for 30 min.Transfer to a low temperature stirred reactor (temperature control at -4 ° C ~ 2 ° C), add 3mol / l hydrochloric acid 36ml to adjust the pH of the solution to 8.5, stir the reaction at low temperature for 30min, form a liquid.8.05 ml (8.25 mmol) of methacryloyl chloride was dissolved in 50 ml of anhydrous chloroform, and stirred at 300 r/min for 30 min to form a B solution.Transfer liquid B to a pear-shaped separatory funnel, and control the rate of 2 drops/second to be added to the solution A. The addition is completed in about two hours.The reaction was continued at -4 ° C to 2 ° C for 2 h to obtain a colorless transparent solution.The water was removed at 55 ° C, 60 mbar for 1 h to give a white powdery solid.The white solid was washed with chromatographically pure methanol (30 ml) (wash once), repeatedly shaken, uniformly mixed, and the upper layer liquid was extracted.The methanol was further removed by rotary evaporation at 45 ° C, 100 mbar to obtain a pale yellow oily liquid, which was dried under vacuum at 50 ° C overnight.A white powder (AEMA) was obtained in a yield of 49%.
References:
Chinese Academy Of Sciences Tsingtao Biological Energies And Process Institute;Shou Qinghui;Ma Gaoqi;Liang Xiangfeng;Luo Xitao;Li Min CN108276303, 2018, A Location in patent:Paragraph 0035
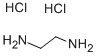
333-18-6
234 suppliers
$14.00/25g
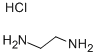
18299-54-2
80 suppliers
inquiry
![Phenol, 2,2'-[1,2-ethanediylbis(nitrilomethylidyne)]bis[6-(1,1-dimethylethyl)-](/CAS/20210305/GIF/72138-54-6.gif)