
Calcium chloride synthesis
- Product Name:Calcium chloride
- CAS Number:10043-52-4
- Molecular formula:CaCl2
- Molecular Weight:110.98
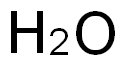
7732-18-5
507 suppliers
$13.50/100ML

10043-52-4
964 suppliers
$5.00/100g
Yield:-
Reaction Conditions:
in methanol;
Steps:
12 Example 12
The gel was immersed in a mixed solution of methanol/water=1/1, and when the fluorescence quantum yield was measured while calcium chloride was being added, it was found that as calcium chloride was added, the fluorescence quantum yield increased, and the gel functioned as a calcium sensor.
References:
US2016/169920,2016,A1