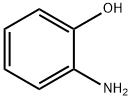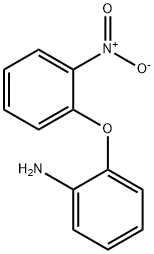
(2-Aminophenyl)-2-nitrophenyl ether synthesis
- Product Name:(2-Aminophenyl)-2-nitrophenyl ether
- CAS Number:91973-79-4
- Molecular formula:C12H10N2O3
- Molecular Weight:230.22
Yield:91973-79-4 90%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 20; for 19 h;Inert atmosphere;
Steps:
3
5.00g in a 200ml three-necked flask under an argon atmosphere2-fluoronitrobenzene is dissolved in 50 ml of anhydrous dimethylformamide (THF).It was then cooled to about 0 °C. Then, 3.86 g of 2-aminophenol and 2.83 g of sodium hydride (60% in oil) were added thereto. Stir about 1 hour at about 0 ° CAfter the time, carry out stirring for about 18 hours while slowly bringing the temperatureReturn to room temperature. After the reaction is completed, water is added at about 0 ° C.It was extracted with dichloromethane (CH 2 Cl 2 ). With magnesium sulfate (MgSO4)After drying, the solvent was distilled and removed under pressure. The crude product thus obtained was separated by silica gel column chromatography to give 7.34 g (yield: 90%)Intermediate K.
References:
CN109553634,2019,A Location in patent:Paragraph 0219-0222; 0224
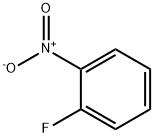
1493-27-2
514 suppliers
$5.00/10g

91973-79-4
3 suppliers
inquiry
2217-65-4
40 suppliers
$21.00/100mg

91973-79-4
3 suppliers
inquiry
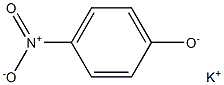
1124-31-8
2 suppliers
inquiry

91973-79-4
3 suppliers
inquiry
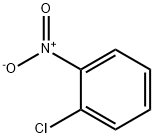
88-73-3
44 suppliers
$16.00/25g

91973-79-4
3 suppliers
inquiry