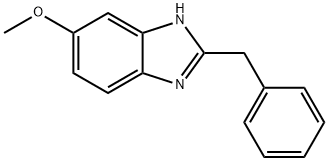
2-BENZYL-5-METHOXY-1H-BENZO[D]IMIDAZOLE synthesis
- Product Name:2-BENZYL-5-METHOXY-1H-BENZO[D]IMIDAZOLE
- CAS Number:40608-76-2
- Molecular formula:C15H14N2O
- Molecular Weight:238.28
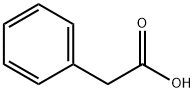
103-82-2
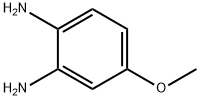
102-51-2
![2-BENZYL-5-METHOXY-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/40608-76-2.gif)
40608-76-2
GENERAL METHOD: Under stirring conditions, 4-methoxy-o-phenylenediamine (1.85 mmol) was dissolved in xylene (10 mL), followed by the addition of phenylacetic acid (2.77 mmol) and boric acid (0.185 mmol). The reaction mixture was refluxed for 16 hours. After completion of the reaction, it was cooled to room temperature, the reaction mixture was concentrated under reduced pressure and diluted with ethyl acetate (50 mL). The organic phase was washed with saturated sodium bicarbonate solution (2 x 50 mL), dried over anhydrous sodium sulfate and subsequently concentrated under reduced pressure. Purification of the residue by silica gel column chromatography (eluent: 10-15% hexane solution of ethyl acetate) afforded 2-benzyl-5-methoxy-1H-benzo[d]imidazole (3u) in 67% yield; white solid; melting point 109-111 °C; IR (KBr) 3009, 2847, 1510, 1466, 1410, 1248, 1175, 1029, 775 cm^-1; 1H NMR (400 MHz, DMSO-d6) δ 12.04-12.14 (m, 1H), 7.18-7.44 (m, 6H), 6.91-7.07 (m, 1H), 6.70-6.79 (m, 1H), 4.12 (s, 2H), 3.75 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 155.5, 137.9, 128.8, 128.5, 126.6, 118.7, 110.1, 101.3, 94.5, 55.4, 34.9; HRMS calculated value of C15H14N2O m/z 238.1182, measured value 238.1180.

103-82-2
0 suppliers
$27.60/100g

102-51-2
215 suppliers
$14.00/1g
![2-BENZYL-5-METHOXY-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/40608-76-2.gif)
40608-76-2
28 suppliers
$47.00/100mg
Yield:40608-76-2 67%
Reaction Conditions:
with boric acid in 5,5-dimethyl-1,3-cyclohexadiene; for 16 h;Reflux;
Steps:
21 General procedure to synthesis of compounds 3a-y and 5
General procedure: To a stirred solution of benzene-1,2-diamine 1 (1.85 mmol)in xylenes (10 mL) were added carboxylic acid 2 (2.77 mmol)and boric acid (0.185 mmol). The resulting solution wasrefluxed for 16 h. After cooling to room temperature, the reactionwas concentrated under reduced pressure and diluted withEtOAc (50 mL). The organic phase was washed with saturatedNaHCO3 solution (2 50 mL), dried over anhydrous Na2SO4and then concentrated under reduced pressure. The residuewas purified by silica gel flash column chromatography (elutingwith 10-15% Ethyl acetate in hexanes) to afford the title compounds3a-y and 5.6.2.21
2-Benzyl-5-methoxy-1H-benzo[d]imidazole (3u)
Yield 67%; Off white solid; mp 109-111 °C; IR (KBr) 3009, 2847, 1510, 1466, 1410, 1248, 1175, 1029, 775 cm-1; 1H NMR (400 MHz, DMSO-d6) δ 12.04-12.14 (m, 1H), 7.18-7.44 (m, 6H), 6.91 - 7.07 (m, 1H), 6.70-6.79 (m, 1H), 4.12 (s, 2H), 3.75 (s, 3H); 13C NMR (100 MHz, DMSO-d6) δ 155.5, 137.9, 128.8, 128.5, 126.6, 118.7, 110.1, 101.3, 94.5, 55.4, 34.9; HRMS calcd for C15H14N2O m/z 238.1182, found 238.1180.
References:
Boggu, PullaReddy;Venkateswararao, Eeda;Manickam, Manoj;Kwak, Dajin;Kim, Youngsoo;Jung, Sang-Hun [Bioorganic and Medicinal Chemistry,2016,vol. 24,# 8,p. 1872 - 1878]

2019-10-5
1 suppliers
inquiry
![2-BENZYL-5-METHOXY-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/40608-76-2.gif)
40608-76-2
28 suppliers
$47.00/100mg
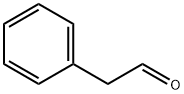
122-78-1
331 suppliers
$10.00/5g

102-51-2
215 suppliers
$14.00/1g
![2-BENZYL-5-METHOXY-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/40608-76-2.gif)
40608-76-2
28 suppliers
$47.00/100mg
16133-49-6
170 suppliers
$7.00/1g
![2-BENZYL-5-METHOXY-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/40608-76-2.gif)
40608-76-2
28 suppliers
$47.00/100mg
103-80-0
322 suppliers
$13.00/25g
![2-BENZYL-5-METHOXY-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/40608-76-2.gif)
40608-76-2
28 suppliers
$47.00/100mg