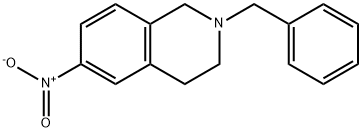
2-benzyl-6-nitro-1,2,3,4-tetrahydroisoquinoline synthesis
- Product Name:2-benzyl-6-nitro-1,2,3,4-tetrahydroisoquinoline
- CAS Number:208589-95-1
- Molecular formula:C16H16N2O2
- Molecular Weight:268.31

124-63-0
0 suppliers
$10.00/5g

100-46-9
502 suppliers
$5.00/5G

186390-74-9
21 suppliers
inquiry

208589-95-1
19 suppliers
inquiry
Yield:208589-95-1 7.76 g (96%)
Reaction Conditions:
with triethylamine in dichloromethane;
Steps:
1.1 Step 1
Step 1 Preparation of N-benzyl-6-nitro-1,2,3,4-tetrahydroisoquinoline A solution of 2-[2-(hydroxymethyl)-5-nitrophenyl]ethanol (5.95 g, 30.17 mmol, J. Org. Chem., 1998, 63, 4116-4119) and triethylamine (10.51 mL, 75.42 mmol) in dry CH2Cl2 (121 mL) at 0° C. under N2 is treated with methanesulfonyl chloride (5.37 mL, 69.39 mmol) dropwise and stirred at 0° C. for 30 mins. The mixture is then washed with 1M aqueous HCl (100 mL), saturated aqueous NaHCO3 (100 mL) and brine (100 mL), dried over Na2SO4 and concentrated under reduced pressure to give the dimesylate intermediate [Rf=0.77 by TCL (MeOH/CHCl3, 10/90)] as a partially crystalline oil. To a solution of this oil in CH2Cl2 (151 mL) under N2 is added benzylamine (16.48 mL, 150.8 mmol), and the resulting mixture is stirred at ambient temperature for 22 hrs and is then washed with water (100 mL) and brine (100 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude product is purified by silica gel chromatography (350 g, 230-400 mesh), eluding with EtOAc/heptane (10/90), to give 7.76 g (96%) of the title compound [Rf=0.43 by TLC (EtOAc/hexane, 25/75)] as a light yellow solid, mp 76-78° C.
References:
US2002/133021,2002,A1

208589-94-0
0 suppliers
inquiry

208589-95-1
19 suppliers
inquiry