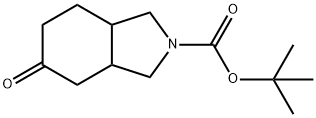
2-Boc-5-oxo-octahydro-isoindole synthesis
- Product Name:2-Boc-5-oxo-octahydro-isoindole
- CAS Number:203661-68-1
- Molecular formula:C13H21NO3
- Molecular Weight:239.31
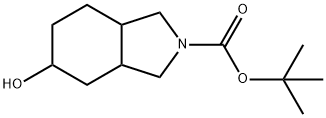
203661-67-0
26 suppliers
$359.00/1g

203661-68-1
31 suppliers
$253.00/250mg
Yield:203661-68-1 100%
Reaction Conditions:
with phthalic acid dimethyl ester in dichloromethane at 0 - 20;
Steps:
7.3 Step 3) Tert-butyl 5-oxohexahydro-1H-isoindole-2(3H)-carboxylate
At 0 ° C, tert-butyl 5-hydroxyhexahydro-1H-isoindole-2(3H)-carboxylate (48.98 g, 203.0 mmol) was dissolved in DCM (500 mL) and DMP (103.31 g, 243.6 mmol) was added in portions. After addition was complete, the reaction was continued at 0 ° C for 0.5 hours and then allowed to move to room temperature and stir overnight. The reaction system was cooled to 0 ° C, then saturated NaHCO 3 solution and saturated Na2S 2 O 3 solution (v / v, 1/1, 500 mL) were added and the mixture was stirred at 0 ° C for 0.5 hours and filtered. The filter cake was rinsed with DCM (250 mL x 3) and the separated aqueous layer was extracted with DCM (250 mL x 3), the combined organic phases were washed with brine (500 mL), dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate (v / v) = 5/1) to give the title compound as a yellow oil (48.58 g, 100%).
References:
CN104672250,2017,B Location in patent:Paragraph 0566; 0567; 0568

79-37-8
490 suppliers
$17.67/10gm:

203661-67-0
26 suppliers
$359.00/1g

203661-68-1
31 suppliers
$253.00/250mg
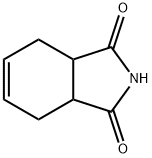
85-40-5
164 suppliers
$7.00/10g

203661-68-1
31 suppliers
$253.00/250mg
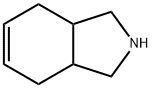
10533-30-9
48 suppliers
$45.00/50mg

203661-68-1
31 suppliers
$253.00/250mg

24424-99-5
871 suppliers
$13.50/25G

203661-68-1
31 suppliers
$253.00/250mg