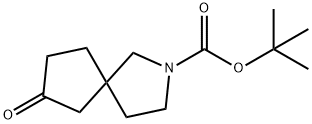
2-Boc-7-oxo-2-Azaspiro[4.4]nonane synthesis
- Product Name:2-Boc-7-oxo-2-Azaspiro[4.4]nonane
- CAS Number:1319716-42-1
- Molecular formula:C13H21NO3
- Molecular Weight:239.31
![7-Hydroxy-2-aza-spiro[4.4]nonane-2-carboxylic acid tert-butyl ester](/CAS/GIF/1319716-41-0.gif)
1319716-41-0
![2-Boc-7-oxo-2-Azaspiro[4.4]nonane](/CAS/20150408/GIF/1319716-42-1.gif)
1319716-42-1
7-Hydroxy-2-azaspiro[4.4]nonane-2-carboxylic acid tert-butyl ester (2.10 g, 8.70 mmol) was used as starting material and suspended in anhydrous dichloromethane (25 mL). Dess-Martin oxidizer (4.43 g, 10.44 mmol) was added to the suspension at 0 °C and under nitrogen protection. Subsequently, the reaction mixture was warmed to room temperature and stirred overnight. Upon completion of the reaction, the mixture was cooled to 0 °C, diluted by the addition of dichloromethane (50 mL), and then quenched by the addition of saturated aqueous sodium bicarbonate (30 mL) and saturated aqueous sodium thiosulfate (30 mL), in that order. The organic phase was separated and washed again with a mixture of saturated aqueous sodium bicarbonate solution (15 mL) and saturated aqueous sodium thiosulfate solution (15 mL). The aqueous phase was back-extracted with dichloromethane (50 mL × 3). All organic phases were combined, washed with saturated brine (50 mL), dried over anhydrous sodium sulfate, and filtered. The residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate, v/v = 3/1) to afford tert-butyl 7-oxo-2-azaspiro[4.4]nonane-2-carboxylate as a yellow liquid (1.30 g, 62% yield). Mass spectrum (ESI, positive ion mode) m/z: 184.2 [(M-C4H8)+ H]+; 1H NMR (600 MHz, CDCl3) δ (ppm): 3.56-3.36 (m, 2H), 3.36-3.24 (m, 2H), 2.39-2.32 (m, 2H), 2.32-2.19 (m, 2H), 2.04 -1.91 (m, 2H), 1.90-1.82 (m, 2H), 1.47 (s, 9H).
![7-Hydroxy-2-aza-spiro[4.4]nonane-2-carboxylic acid tert-butyl ester](/CAS/GIF/1319716-41-0.gif)
1319716-41-0
38 suppliers
$45.00/5mg
![2-Boc-7-oxo-2-Azaspiro[4.4]nonane](/CAS/20150408/GIF/1319716-42-1.gif)
1319716-42-1
55 suppliers
$90.00/25mg
Yield: 62%
Reaction Conditions:
with Dess-Martin periodane in dichloromethane at 0 - 20;Inert atmosphere;
Steps:
15.5 Step 5) fey -butyl 7-oxo-2-azaspiro[4.4]nonane-2-carboxylate
[382] To a suspension of terf-butyl 7-hydroxy-2-azaspiro[4.4]nonane-2-carboxylate (2.10 g, 8.70 mmol) in anhydrous DCM (25 mL) was added Dess-Martin (4.43 g, 10.44 mmol) at 0 °C under nitrogen atmosphere, and then the mixture was moved to room temperature and stirred overnight. The mixture was cooled down to 0 °C and added DCM (50 mL), then added saturated NaHC03 aqueous solution (30 mL) and saturated Na2S2( aqueous solution (30 mL). The separated organic phase was washed with a mixture of saturated NaHC03 aqueous solution (15 mL) and saturated Na2S203 aqueous solution (15 mL) again. The combined aqueous phases were extracted with DCM (50 mLx3). The combined organic phases were washed with saturated brine (50 mL), dried over anhydrous Na2S04, filtered and concentrated in vacuo. The residue was purified by silica gel column chromatography (PE/EtOAc (v/v) = 3/1) to give the title compound as yellow liquid (1.30 g, 62%). MS (ESI, pos. ion) m z: 184.2 [(M-C4)+H]+; 'H NMR (600 MHz, CDCI3) δ (ppm): 3.56-3.36 (m, 2H), 3.36-3.24 (m, 2H), 2.39-2.32 (m, 2H), 2.32-2.19 (m, 2H), 2.04-1.91 (m, 2H), 1.90-1.82 (m, 2H), 1.47 (s, 9H).
References:
CALITOR SCIENCES, LLC;SUNSHINE LAKE PHARMA CO., LTD.;XI, Ning;LI, Minxiong;HU, Haiyang;WANG, Tingjin WO2015/94803, 2015, A1 Location in patent:Paragraph 382

1319716-35-2
0 suppliers
inquiry
![2-Boc-7-oxo-2-Azaspiro[4.4]nonane](/CAS/20150408/GIF/1319716-42-1.gif)
1319716-42-1
55 suppliers
$90.00/25mg
![2-Azaspiro[4.4]non-7-ene-2-carboxylic acid, 1-oxo-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1319716-36-3.gif)
1319716-36-3
2 suppliers
inquiry
![2-Boc-7-oxo-2-Azaspiro[4.4]nonane](/CAS/20150408/GIF/1319716-42-1.gif)
1319716-42-1
55 suppliers
$90.00/25mg
85909-08-6
238 suppliers
$6.00/1g
![2-Boc-7-oxo-2-Azaspiro[4.4]nonane](/CAS/20150408/GIF/1319716-42-1.gif)
1319716-42-1
55 suppliers
$90.00/25mg

24424-99-5
871 suppliers
$13.50/25G
![2-Boc-7-oxo-2-Azaspiro[4.4]nonane](/CAS/20150408/GIF/1319716-42-1.gif)
1319716-42-1
55 suppliers
$90.00/25mg