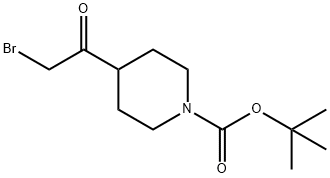
2-bromo-1-(1-Boc-piperidin-4-yl)ethanone synthesis
- Product Name:2-bromo-1-(1-Boc-piperidin-4-yl)ethanone
- CAS Number:301221-79-4
- Molecular formula:C12H20BrNO3
- Molecular Weight:306.2
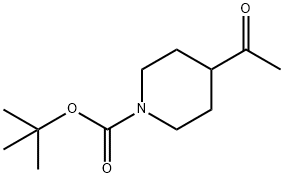
206989-61-9

301221-79-4
The general procedure for the synthesis of N-Boc-4-(bromoacetyl)piperidine from 1-N-BOC-4-acetylpiperidine was as follows: to a suspension of tert-butyl 4-ethylpiperidine-1-carboxylate (Intermediate B5; 2.87 g, 12.6 mmol) in THF (30 mL) cooled to -78 °C, a THF solution of 1 M bis(trimethylmethylsilyl)lithium amide was slowly added (13.3 mL), and the addition time was controlled within 20 min. The reaction mixture was stirred at -78 °C for 1 h. Trimethylsilyl chloride (1.74 mL, 13.7 mmol) was added. Stirring was continued at 0 °C for 30 min, after which the reaction solution was cooled again to -78 °C and bromine (0.645 mL, 12.6 mmol) was added slowly. After the reaction mixture was naturally warmed to room temperature, the reaction was quenched by pouring into a mixture of 10% Na2S2O3 solution (20 mL) and saturated NH4Cl solution (20 mL). The organic phase was extracted with EtOAc (2×80 mL) and the organic layers were combined, which were treated to afford the target product N-Boc-4-(bromoacetyl)piperidine in a yield of 3.69 g in 96%.

206989-61-9
217 suppliers
$10.00/1g

301221-79-4
109 suppliers
$33.00/250mg
Yield:301221-79-4 96%
Reaction Conditions:
Stage #1: 1-(tert-Butoxycarbonyl)piperidin-4-yl methyl ketonewith chloro-trimethyl-silane;lithium hexamethyldisilazane in tetrahydrofuran at -78 - 0;
Stage #2: with bromine in tetrahydrofuran at -78 - 20;
Steps:
INTERMEDIATE B6 tert-Butyl 4-(bromoacetyl)piperidine-l-carboxylate To a cooled (-78 0C) suspension of tert-butyi 4-acetylpiperidine-l-carboxylate (Intermediate B5; 2.87 g, 12.6 mmol) in THF (30 mL) was added 1 M lithium bis(trimethylsilyl)amide in THF (13.3 mL) over 20 min. The mixture was stirred for 1 h before the addition of trimethylsilyl chloride (1.74 mL, 13.7 mmol). After stirring at 0 0C for 30 min, the solution was cooled to -78 0C and bromine (0.645 mL, 12.6 mmol) was added. The mixture was allowed to reach r.t. and then poured into a solution of 10% Na2S2O3 (20 mL) and saturated NH4Cl (20 mL). Extraction with EtOAc (2 x 80 mL) gave the title compound. Yield 3.69 g (96%).
References:
WO2009/150144,2009,A1 Location in patent:Page/Page column 139
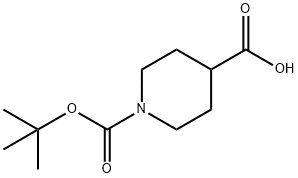
84358-13-4
379 suppliers
$5.00/5g

301221-79-4
109 suppliers
$33.00/250mg
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
182 suppliers
$6.00/100mg

301221-79-4
109 suppliers
$33.00/250mg