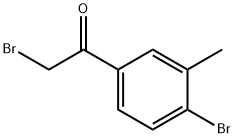
2-broMo-1-(4-broMo-3-Methylphenyl)ethanone synthesis
- Product Name:2-broMo-1-(4-broMo-3-Methylphenyl)ethanone
- CAS Number:3114-08-7
- Molecular formula:C9H8Br2O
- Molecular Weight:291.97
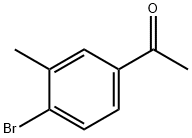
37074-40-1
110 suppliers
$51.00/250mg

3114-08-7
18 suppliers
$227.00/1g
Yield:3114-08-7 60%
Reaction Conditions:
with copper(ll) bromide in ethyl acetate at 80;Inert atmosphere;
Steps:
2-bromo-l-(4-bromo-3-methylphenyl)ethanone
In a two necked round bottom flask equipped with a reflux condenser under an argon atmosphere was added CuBr2 (700 mg, 3.14 mmol) and EtOAc (8 mL). The suspension was stirred during 20 minutes. The 4-bromo-3- methylacetophenonone (335 mg, 1.57 mmol) pre-dissolved in EtOAc (8 mL) was added to the mixture and heated at 80°C during one hour. A new equivalent of CuBr2 (330 mg, 1.57 mmol) was added and the reaction was stirred at 80°C overnight. The suspension was cooled down, filtered through a Celite pad and washed with EtOAc. The filtrate was washed with a saturated NaHCO3 solution. The organic layer was dried over Na2SO4, filtered and evaporated under vacuum. Purification by silica gel flash chromatography (PE/EtOAc 6: 1) afforded the product (273 mg, 60%) as a white oil. NMR (300 MHz, CDCb) δ 7.85 - 7.80 (m, 1H, Har), 7.68 - 7.58 (m, 2H, Har), 4.40 (s, 2H, CH2), 2.46 (s, 3H, CH3). 13C NMR (75 MHz, CDCb) δ 191.0 (C), 139.3 (C), 133.3 (CH), 133.2 (C), 132.0 (C), 131.2 (CH), 127.9 (CH), 30.9 (CH2), 23.3 (CH3). HRMS (ESI) [M+H]+ C9H9Br2O: Calcd. 312.8834 found 312.8838.
References:
WO2015/1024,2015,A1 Location in patent:Page/Page column 69
97674-02-7
219 suppliers
$15.00/1g
202865-85-8
221 suppliers
$8.00/5g

3114-08-7
18 suppliers
$227.00/1g

202865-85-8
221 suppliers
$8.00/5g

3114-08-7
18 suppliers
$227.00/1g
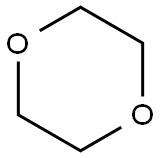
123-91-1
656 suppliers
$17.00/25g

37074-40-1
110 suppliers
$51.00/250mg

3114-08-7
18 suppliers
$227.00/1g
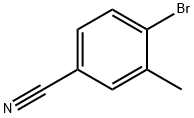
41963-20-6
179 suppliers
$15.00/1g

3114-08-7
18 suppliers
$227.00/1g