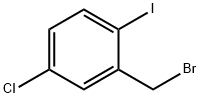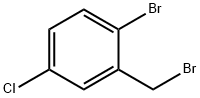
2-Bromo-1-bromomethyl-5-chlorobenzene synthesis
- Product Name:2-Bromo-1-bromomethyl-5-chlorobenzene
- CAS Number:66192-24-3
- Molecular formula:C7H5Br2Cl
- Molecular Weight:284.38
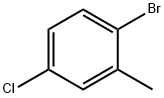
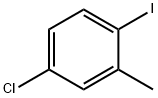
14495-51-3

66192-24-3
The general procedure for the synthesis of 2-bromo-5-chlorobenzyl bromide from 2-bromo-5-chlorotoluene was as follows: 20.5 g (100 mmol) of 2-bromo-5-chlorotoluene was dissolved in 200 mL of carbon tetrachloride, followed by the addition of 0.2 g (1 mmol) of AIBN (azobisisobutyronitrile) and 19.6 g (110 mmol) of N-bromosuccinimide (NBS). The resulting mixture was refluxed for 2 h and then cooled to room temperature. The reaction mixture was sequentially washed twice with 50 mL of water, then with 50 mL of brine solution and finally dried with anhydrous magnesium sulfate. The solvent was removed by distillation under reduced pressure to give an oil containing a small amount of unreacted material. The oily substance was dissolved in 20 mL of hexane and recrystallized at room temperature to give 22.7 g (80% yield) of 2-bromo-1-bromomethyl-5-chlorobenzene. The product was characterized by 1H NMR (300 MHz, CDCl3): δ 7.50 (d, 1H, J = 8.7 Hz), 7.45 (d, 1H, J = 2.4 Hz), 7.15 (dd, 1H, J = 8.7 Hz, 2.4 Hz), 4.53 (s, 2H).

14495-51-3
261 suppliers
$10.00/5g

66192-24-3
127 suppliers
$9.00/1g
Yield:66192-24-3 80%
Reaction Conditions:
with NBS;(E)-2,2'-(diazene-1,2-diyl)bis( 2-methylpropanenitrile) in Carbon tetrachloride; for 2 h;Heating / reflux;
Steps:
2.1
20.5 g (100 mmol) of 2-bromo-5-chlorotoluene was dissolved in 200 ml of carbon tetrachloride, 0.2 g (1 mmol) of AIBN and 19.6 g (110 mmol) of N-bromosuccinimide were added thereto, the resulting mixture was refluxed for 2 hours and cooled to room temperature. The reaction mixture was washed with 50 ml of water twice and with 50 ml of a brine solution, dried over anhydrous MgS04, and distilled under a reduced pressure to obtain an oil containing a small amount of the starting material. The oil was dissolved in 20 ml of hexane, and recrystallized at room temperature to obtain ' 22.7 g (yield 80%) of 2-bromo-l-bromomethyl-5-chlorobenzene. 1H NMR (300 MHz, CDC13) d 7.50 (d, 1H, J= 8.7 Hz), 7.45 (d, 1H, J= 2.4 Hz), 7.15 (dd, 1H, J= 8.7 Hz, 2.4 Hz), 4.53 (s, 2H)
References:
WO2005/123054,2005,A1 Location in patent:Page/Page column 25
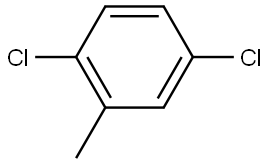
19398-61-9
138 suppliers
$10.00/5G

66192-24-3
127 suppliers
$9.00/1g
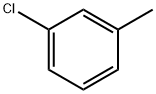
108-41-8
274 suppliers
$19.00/10g

66192-24-3
127 suppliers
$9.00/1g