
2-BroMo-1-iodo-3-(trifluoroMethyl)benzene synthesis
- Product Name:2-BroMo-1-iodo-3-(trifluoroMethyl)benzene
- CAS Number:1049731-01-2
- Molecular formula:C7H3BrF3I
- Molecular Weight:350.9
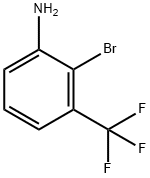
58458-10-9
88 suppliers
$21.00/1g

1049731-01-2
25 suppliers
$75.00/25mg
Yield:1049731-01-2 10.0 g
Reaction Conditions:
Stage #1: 2-bromo-3-(trifluoromethyl)anilinewith sulfuric acid in water at 0; for 0.5 h;
Stage #2: with sodium nitrite in water at 0; for 0.5 h;
Stage #3: with iodine;potassium iodide in water at 0 - 40;
Steps:
109.1 2-Bromo-3-trifluoromethyl-iodobenzene
Step 1:
2-Bromo-3-trifluoromethyl-iodobenzene
2-Bromo-3-trifluoromethylaniline (8 g, 33.33 mmol) is suspended in water (90 mL) and cooled to 0° C. 96% H2SO4 (26.7 mL, 13.7 mmol) is added and the mixture stirred for 30 minutes. NaNO2 (2.41 g, 35 mmol) is dissolved in a small quantity of water and added dropwise at 0° C.
The mixture is stirred for 30 minutes then potassium iodide (9.24 g, 55.66 mmol) and iodine (9.31 g, 36.66 mmol) in water are added dropwise with cooling.
The mixture is stirred until gas evolution ceases then warmed to 40° C. and allowed to cool to room temperature.
The mixture is shaken with excess aqueous Na2SO3 solution and extracted with ethyl acetate.
The organic extracts are evaporated and the residue purified by flash chromatography (eluent:cyclohexane) to give the title compound (Yield 10.0 g)
GC (GC METHOD 1): tR=8.40 min; Mass spectrum (EI+): m/z=350 [M]+.
References:
US2013/324514,2013,A1 Location in patent:Paragraph 0567
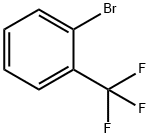
392-83-6
332 suppliers
$6.00/10g

1049731-01-2
25 suppliers
$75.00/25mg