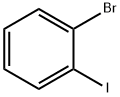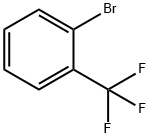
2-Bromobenzotrifluoride synthesis
- Product Name:2-Bromobenzotrifluoride
- CAS Number:392-83-6
- Molecular formula:C7H4BrF3
- Molecular Weight:225.01
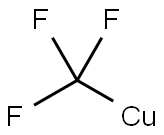
88-17-5
372 suppliers
$14.00/25g

392-83-6
330 suppliers
$6.00/10g
Yield:392-83-6 89%
Reaction Conditions:
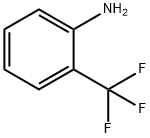
Stage #1:2-(trifluoromethyl)benzenamine with hydrogen bromide;sodium nitrite in water at 0 - 5; for 0.333333 h;
Stage #2: with hydrogen bromide;copper(I) bromide in water at 20; for 0.333333 h;Sandmeyer Reaction;
Steps:
2.1 (1) Sandemeyer bromination reaction
While stirring slowly, 308 g (1.9 mol) of o-trifluoromethylaniline was added to 970 mL of hydrobromic acid (40%) with stirring After cooling with ice bath to 0 ° C. 140 g (2.03 mol) of sodium nitrite was dissolved in 325 mL of water and slowly added dropwise with stirring Reaction system, and the reaction temperature does not exceed 5 ° C. After dripping, continue stirring for 20 min, keep warm. To 2 L four mouth The flask was charged with 23.5 g (0.34 mol) of cuprous bromide, 65 mL of hydrobromic acid (40%), stirred vigorously at room temperature, and the above diazonium Poured into the four reaction bottles, the reaction has a large amount of gas generated, the diazonium solution added after the continuous stirring 20 min, liquid, the lower The substrate was washed with alkali and washed with water. The product was simply distilled to obtain a yellow liquid product o-bromotrifluorotoluene 386 g, GC content: 99% yield: 89%. The upper aqueous phase is recyclable with hydrobromic acid and cuprous bromide.
References:
Zhejiang Weihua Chemical Co., Ltd.;Chen Jinghua;Wu Jiangwei;Bu Luzhou;Li Junqi;Chang Xuanchao;Zhang Quanquan;Zhao Hongying CN106905104, 2017, A Location in patent:Paragraph 0020
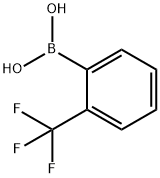
1423-27-4
318 suppliers
$6.00/1g

392-83-6
330 suppliers
$6.00/10g
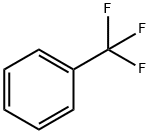
98-08-8
298 suppliers
$9.00/1g

392-83-6
330 suppliers
$6.00/10g
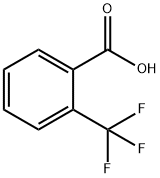
433-97-6
344 suppliers
$10.00/5g

392-83-6
330 suppliers
$6.00/10g