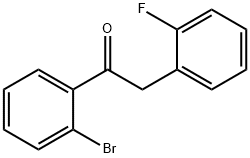
2'-BROMO-2-(2-FLUOROPHENYL)ACETOPHENONE synthesis
- Product Name:2'-BROMO-2-(2-FLUOROPHENYL)ACETOPHENONE
- CAS Number:898784-61-7
- Molecular formula:C14H10BrFO
- Molecular Weight:293.13
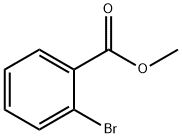
610-94-6
314 suppliers
$13.00/25g
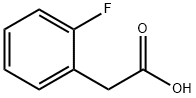
451-82-1
351 suppliers
$6.00/5g

898784-61-7
11 suppliers
$100.00/50mg
Yield:898784-61-7 37%
Reaction Conditions:
with sodium hexamethyldisilazane in tetrahydrofuran at -70 - 0; for 0.833333 h;Inert atmosphere;
Steps:
34A 1-(2-Bromophenyl)-2-(2-fluorophenyl)ethanone
Example 34A
1-(2-Bromophenyl)-2-(2-fluorophenyl)ethanone
15.0 g (69.8 mmol) of 2-methyl bromobenzoate and 11.8 g (76.7 mmol) of 2-fluorophenylacetic acid were put in THF (278 ml) under argon atmosphere at -70° C. and 174 ml of a 1M solution of sodium hexamethyldisilazane in THF was added dropwise in the space of 20 min.
The reaction mixture was heated to 0° C., stirred for 30 min at this temperature and 1N hydrochloric acid (278 ml) was added.
After 1 h of vigorous stirring with evolution of gas (CO2 cleavage), the reaction mixture was extracted with ethyl acetate (500 ml).
The organic phase was washed twice with saturated aqueous sodium hydrogen carbonate solution, once with water and once with saturated aqueous sodium chloride solution.
After drying and removal of the solvent in the rotary evaporator, 16.8 g of residue was obtained (55% purity).
The residue was dissolved in THF (140 ml), 1N sodium hydroxide solution (70 ml) was added and it was stirred for 4 h at RT, in order to saponify excess ester.
The THF was removed in the rotary evaporator, the aqueous phase was extracted with diethyl ether and the organic phase was washed with saturated aqueous sodium hydrogen carbonate solution and saturated aqueous sodium chloride solution.
After drying and removal of the solvent, 12.2 g of residue was obtained (approx. 80% purity).
The residue was dissolved in THF (100 ml), 1N sodium hydroxide solution (40 ml) was added and it was stirred overnight at RT.
The THF was removed in the rotary evaporator, the aqueous phase was extracted with diethyl ether and the organic phase was washed with saturated aqueous sodium hydrogen carbonate solution and saturated aqueous sodium chloride solution.
After drying and removal of the solvent, 7.90 g (37% of theor.) of the title compound was isolated.
1H-NMR (400 MHz, DMSO-d6): δ [ppm]=4.35 (s, 2H), 7.14-7.22 (m, 2H), 7.30-7.39 (m, 2H), 7.41-7.47 (m, 1H), 7.49-7.55 (m, 1H), 7.70-7.78 (m, 2H).
References:
US2013/338137,2013,A1 Location in patent:Paragraph 1567; 1568; 1569