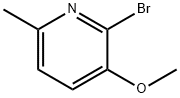
2-Bromo-3-methoxy-6-methylpyridine synthesis
- Product Name:2-Bromo-3-methoxy-6-methylpyridine
- CAS Number:24207-22-5
- Molecular formula:C7H8BrNO
- Molecular Weight:202.05
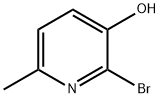
23003-35-2

74-88-4

24207-22-5
General procedure for the synthesis of 2-bromo-3-methoxy-6-methylpyridine from 2-bromo-3-hydroxy-6-methylpyridine and iodomethane: 2-bromo-3-hydroxy-6-methylpyridine (7.89 g, 42.0 mmol), potassium carbonate (11.60 g, 83.9 mmol), and iodomethane (8.93 g, 62.9 mmol, 3.92 mL) were mixed in acetone (100 mL) in acetone was stirred. The reaction mixture was heated to reflux overnight. Upon completion of the reaction, the mixture was filtered, the solvent was evaporated and purified by silica gel column chromatography (eluent: hexane/ethyl acetate, 95:5 to 9:1) to afford the target product 2-bromo-3-methoxy-6-methylpyridine as a white solid (7.49 g, 88.3% yield).1H NMR (500 MHz, CDCl3): δ 2.46 (s, 3H), 3.87 (s, 3H), 3.87 (s, 3H). 3.87 (s, 3H), 7.04 (d, J = 8.0 Hz, 1H), 7.10 (d, J = 8.0 Hz, 1H).

23003-35-2
125 suppliers
$13.00/250mg

74-88-4
357 suppliers
$15.00/10g

24207-22-5
81 suppliers
$45.00/50mg
Yield:24207-22-5 88.3%
Reaction Conditions:
with potassium carbonate in acetone;
Steps:
9.b Example 9
b. 2-Bromo-3-methoxy-6-methyl-pyridine. A stirred mixture of 2-bromo-3-hydroxy-6-methyl-pyridine (7.89 g, 42.0 mmol), potassium carbonate (11.60 g, 83.9 mmol) and iodomethane (8.93 g, 62.9 mmol, 3.92 mL) in acetone (100 mL) was heated under reflux overnight. The mixture was filtered, evaporated and purified on silica gel (hexane:ethyl acetate, 95:5 to 9:1) to give the desired product as a white solid (7.49 g, 88.3%). 1H NMR (500 MHz, CDCl3): δ2.46 (s, 3 H), 3.87 (s, 3 H), 7.04 (s, 2 H).
References:
US2003/83357,2003,A1
1121-78-4
286 suppliers
$6.00/5g

24207-22-5
81 suppliers
$45.00/50mg