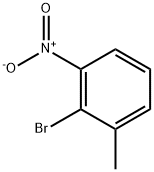
2-Bromo-3-nitrotoluene synthesis
- Product Name:2-Bromo-3-nitrotoluene
- CAS Number:41085-43-2
- Molecular formula:C7H6BrNO2
- Molecular Weight:216.03
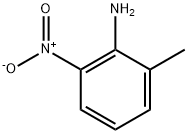
570-24-1

41085-43-2
The general procedure for the preparation of 2-bromo-3-nitrotoluene by diazotization reaction using 2-methyl-6-nitroaniline as starting material was as follows: first, 2-methyl-6-nitroaniline (30.4 g, 0.2 mol) was suspended in water (250 mL) and 40% aqueous hydrobromic acid (100 mL), refluxed for 10 min and then cooled to 0 °C. A solution of sodium nitrite (13.8 g, 0.2 mol) in water (80 mL) was added slowly and dropwise at a temperature below 5 °C. The resulting diazonium salt solution was continued to be stirred at 0-5 °C for 30 min, followed by the slow addition of a stirring mixed solution of cuprous bromide (28.7 g, 0.2 mol) in hydrobromic acid (80 mL) and water (150 mL) at room temperature. The reaction mixture was stirred at room temperature for 30 minutes and then heated and stirred on a steam bath for 1 hour. Upon completion of the reaction, it was washed with saturated sodium bicarbonate solution and brine, dried over magnesium sulfate and concentrated. The residue was purified by column chromatography using petroleum ether as eluent to give 2-bromo-3-nitrotoluene as a light yellow solid (25.9 g, 60% yield).
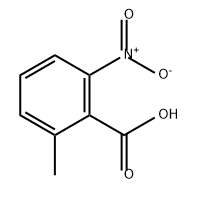
13506-76-8
261 suppliers
$6.00/1g

41085-43-2
199 suppliers
$6.00/1g
Yield: 30%
Reaction Conditions:
with 2.9-dimethyl-1,10-phenanthroline;oxygen;copper (I) acetate;silver sulfate;sodium bromide in dimethyl sulfoxide at 160; for 24 h;Schlenk technique;
Steps:
24 Example 24. Synthesis of 2-nitro-6-methyl bromobenzene.
Silak reaction tube equipped with a magnetic stirrer was charged with 6.2 mg of silver sulfate,36.3 mg of copper acetate, 12.5 mg of 2,9-dimethyl-1,10-o-phenanthroline,36.2 mg of 2-nitro-6-methylbenzoic acid and 30.9 mg of sodium bromide4 mL of dimethyl sulfoxide.The reaction was heated at 160 ° C for 24 hours in the presence of oxygen.After the reaction was completed, distilled water was added to quench the reaction,Extraction with ethyl acetate 3 times, each time 10mL,The combined organic phases are concentrated,13.0 mg of 2-nitro-6-methylbromobenzene was obtained in a yield of 30%.
References:
Nanchang University;Fu Zhengjiang;Cai Hu;Li Zhaojie;Jiang Ligao CN107325002, 2017, A Location in patent:Paragraph 0118

570-24-1
368 suppliers
$6.00/10g

41085-43-2
199 suppliers
$6.00/1g
59907-22-1
23 suppliers
$33.39/250mg

41085-43-2
199 suppliers
$6.00/1g
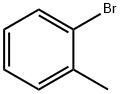
95-46-5
301 suppliers
$10.00/10 g

41085-43-2
199 suppliers
$6.00/1g

95-46-5
301 suppliers
$10.00/10 g
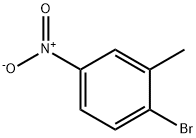
7149-70-4
189 suppliers
$9.00/1g
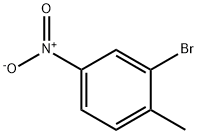
7745-93-9
294 suppliers
$5.00/5g

41085-43-2
199 suppliers
$6.00/1g
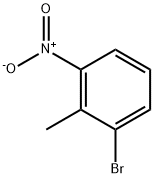
55289-35-5
306 suppliers
$13.00/1g