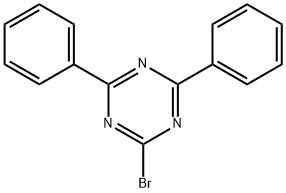
2-BROMO-4,6-DIPHENYL-[1,3,5]TRIAZINE synthesis
- Product Name:2-BROMO-4,6-DIPHENYL-[1,3,5]TRIAZINE
- CAS Number:80984-79-8
- Molecular formula:C15H10BrN3
- Molecular Weight:312.16
14921-00-7

98-80-6
![2-BROMO-4,6-DIPHENYL-[1,3,5]TRIAZINE](/CAS/GIF/80984-79-8.gif)
80984-79-8
Under nitrogen protection, 2,4,6-tribromo-1,3,5-triazine ring (31.78 g, 100.05 mmol) and phenylboronic acid (12.19 g, 100.01 mmol) were added to a 2L reactor, followed by potassium carbonate (1.24 g, 9.00 mmol) and 200 mL of toluene as solvent. After heating the reaction mixture to 70 °C, tetrakis(triphenylphosphine)palladium (1.04 g, 0.90 mmol) was added as a catalyst. Next, 100 mL of distilled water was added and the reaction mixture was stirred and refluxed for 11 hours to ensure complete reaction. Upon completion of the reaction, 70 mL of distilled water was added to quench the reaction. The mixture was separated by filtration under reduced pressure and the solid product was washed with distilled water. Subsequently, recrystallization was carried out using toluene followed by THF solidification and finally further purification by sublimation and recrystallization from toluene afforded the intermediate 2-bromo-4,6-diphenyl-1,3,5-triazine (30.89 g in 84.10% yield).
Yield: 84.1%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);potassium carbonate in water;toluene at 70; for 11 h;Inert atmosphere;
Steps:
1 Preparation of Compound 1-5
Under a nitrogen atmosphere, add 1-4 (31.78 g, 100.05 mmol) to the 2L reactor.1-2 (12.19 g, 100.01 mmol), potassium carbonate (1.24 g, 9.00 mmol), 200 mL of toluene was stirred.The temperature in the reactor was raised to 70°C and Pd(PPh3)4 (1.04 g, 0.90 mmol) was added.100 mL of distilled water was stirred, and the mixture was stirred and refluxed for 11 h to fully react.After adding 70 mL of distilled water to stop the reaction, the mixture was filtered under reduced pressure, and the solid was washed with distilled water, followed by acetone.Recrystallization from toluene, THF, solidification followed by sublimation, toluene recrystallization,Intermediate 1-5 30.89 g was obtained with a yield of 84.10%.
References:
Changchun Hai Purunsi Technology Co., Ltd.;Zhang Hong;Cai Hui CN107879993, 2018, A Location in patent:Paragraph 0048; 0053-0056