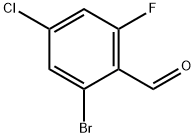
2-BroMo-4-chloro-6-fluorobenzaldehyde synthesis
- Product Name:2-BroMo-4-chloro-6-fluorobenzaldehyde
- CAS Number:1135531-73-5
- Molecular formula:C7H3BrClFO
- Molecular Weight:237.45
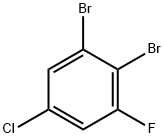
208186-78-1
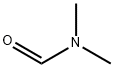
68-12-2

1135531-73-5
Step 1: Isopropylmagnesium chloride (19.2 mL, 1 M in tetrahydrofuran) was slowly added to a mixture of 1,2-dibromo-5-chloro-3-fluorobenzene (5 g, 17.5 mmol) in hexane (12 mL) and tetrahydrofuran (20 mL) at -45 °C. The reaction temperature was maintained at -40°C. Subsequently, N,N-dimethylformamide (6.4 g, 87.5 mmol) was slowly added dropwise to the above reaction mixture and stirred at the same temperature for 30 min. After completion of the reaction, the reaction was quenched with hydrochloric acid (15 mL, 2.0 M). The mixture was gradually warmed to room temperature and diluted with ethyl acetate (80 mL) to separate the organic phase. The organic phase was washed with saturated brine, filtered and the filtrate concentrated under reduced pressure. The residue was purified by fast column chromatography (petroleum ether/ethyl acetate = 10/1) to afford 2-bromo-4-chloro-6-fluorobenzaldehyde (3 g, yield: 73%) as a yellow solid.

208186-78-1
121 suppliers
$19.00/5g

1135531-73-5
82 suppliers
$28.00/100mg
Yield: 95%
Reaction Conditions:
with isopropylmagnesium chloride in tetrahydrofuran;n-heptane at -40; for 0.5 h;Inert atmosphere;
Steps:
B-2.A
ΓΑ1 2-Bromo-4-chloro-6-fluoro-benzaldehydeTo a solution of l,2-dibromo-5-chloro-3-fluoro-benzene (10 g, 34.68 mmol) in heptane (27 ml) was added THF (44 ml) and the mixture was cooled to -45 °C. Then, iPrMgCl (38.14 ml, 38.14 mmol, 1M solution in THF) was added dropwise to the reaction mixture maintaining the temperature between -40 °C to -45° C. The mixture was stirred for 30 minutes at -40 °C before DMF (13.4 ml, 173.4 mmol) was added dropwise to the reaction mixture maintaining the temperature between -45 °C to -20 °C. After stirring for another 15 minutes at -20 °C, it was poured into a mixture of 2N HC1 (20 ml) and ether (50 ml) at 0 °C. The organic layer was separated and the aqueous layer was extracted two times with ether. The combined organic layers were dried with Na2S04 and evaporated in vacuo to obtain the title compound (7.8 g, 95%) as yellow solid.
References:
F. HOFFMANN-LA ROCHE AG;AEBI, Johannes;BINGGELI, Alfred;HERTEL, Cornelia;KONKAR, Anish Ashok;KUEHNE, Holger;KUHN, Bernd;MAERKI, Hans P.;WANG, Haiyan WO2012/101011, 2012, A2 Location in patent:Page/Page column 68-69
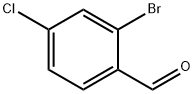
84459-33-6
194 suppliers
$7.00/5g

1135531-73-5
82 suppliers
$28.00/100mg