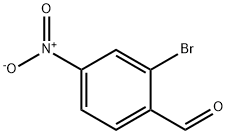
2-Bromo-4-nitrobenzaldehyde synthesis
- Product Name:2-Bromo-4-nitrobenzaldehyde
- CAS Number:5274-71-5
- Molecular formula:C7H4BrNO3
- Molecular Weight:230.02
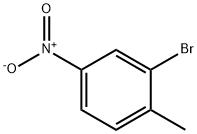
7745-93-9

5274-71-5
GENERAL STEPS: 2-Bromo-1-methyl-4-nitrobenzene (15.0 g, 69.4 mmol) was dissolved in a mixture of peracetic acid (112 mL) and acetic anhydride (105 mL, 1,113 mmol). The reaction mixture was cooled in an ice water bath and concentrated sulfuric acid (15 mL, 281 mmol) was added slowly. Subsequently, chromium (VI) oxide (34.7 g, 347 mmol) was added in batches while the reaction temperature was strictly controlled in the range of 5-10 °C. The reaction mixture was continuously stirred in an ice bath for 1.5 h, during which the temperature needed to be closely monitored, and an ice-acetone bath was used to maintain the temperature at no more than 10 °C if necessary. Upon completion of the reaction, the mixture was poured into a 2 L conical flask containing an appropriate amount of ice, and the total volume was adjusted to 1,500 mL by adding cold water.The precipitate was collected through a Brinell funnel and washed with cold water until nearly colorless. The resulting solid was suspended in cold 2% aqueous Na2CO3 solution (100 mL), stirred thoroughly and the solid was again collected, washed with cold water and partially dried over a Brinell's funnel to give 8.3 g of crude diacetate intermediate. The intermediate (8.3 g) was mixed with ethanol (16 mL), water (16 mL) and concentrated sulfuric acid (2.4 mL) and heated to reflux for 1 hour. After cooling to room temperature, the mixture was transferred to a split funnel containing saturated aqueous K2CO3 solution (50 mL). Fluffy solid formation was observed and identified as non-target product. The aqueous layer was extracted with ethyl acetate (4 x 50 mL), the organic layers were combined and washed with brine (25 mL), dried over MgSO4, filtered and concentrated. Purification by silica gel column chromatography (gradient elution of 5%-15% ethyl acetate in hexane solution) afforded 2-bromo-4-nitrobenzaldehyde (1.41 g, 9% yield) as a colorless fluffy solid.1H NMR (400 MHz, CDCl3) δ 10.41 (s, 1H), 8.51 (d, J = 2.0 Hz, 1H), 8.25 (dd, J = 8.4), 2.1 Hz, 2.1 Hz, 1 Hz. , 2.1 Hz, 1H), 8.06 (d, J = 8.6 Hz, 1H).

7745-93-9
293 suppliers
$5.00/5g

5274-71-5
78 suppliers
$35.00/250mg
Yield: 9%
Reaction Conditions:
Stage #1:2-bromo-4-nitrotoluene with chromium(VI) oxide;sulfuric acid;acetic anhydride in acetic acid at 5 - 10; for 1.5 h;Cooling with ice;
Stage #2: with sulfuric acid in ethanol;water for 1 h;Reflux;
Steps:
12.A Part A. 2-Bromo-4-nitrobenzaldehyde
2-Bromo-1-methyl-4-nitrobenzene (15.0 g, 69.4 mmol) was dissolved inacetic acid (112 mL) and acetic anhydride (105 mL, 1,113 mmol). The mixture was placed in an ice-water bath and concentrated sulfuric acid (15 mL, 281 mmol) was added slowly. Chromium(VI) oxide (34.7 g, 347 mmol) was then added in portionswhile maintaining the temperature of the reaction mixture between 5-10 ° C. The reaction mixture was stirred in an ice-bath for 1.5 h. The internal temperaturehovered between 5-10 °C. It was necessary to monitor the reaction temperature due to a delayed exotherm. At one point it was necessary to place the reaction mixture in an ice-acetone bath for a period of time to keep the temperature from exceeding 10°C. The contents of the flask were then poured into a 2 liter Erlenmeyer flask containing some ice. Cold water was then added to bring the total volume to 1500mL. The solid was collected on a Buchner funnel. The solid was washed with cold water until it was nearly colorless. The solid was suspended in cold 2% aqueous Na2CO3 solution (100 mL) and was thoroughly stirred. The solid was collected on a filter and was washed with cold water and partially dried on the Buchner funnel to give 8.3 grams of the diacetate intermediate.A mixture of 8.3 g of crude diacetate intermediate, ethanol (16 mL), water (16 mL), and conc. H2S04 (2.4mL) was heated at reflux for 1 h. The mixture was cooled to room temperature and the mixture was transferred to a separatory funnel containing saturated aqueous K2C03 (50 mL) solution. A fluffy solid formed. It was subsequently determined that this solid was not the product. The aqueous layer wasextracted with ethyl acetate (4 x 50 mL). The combined organic layers were washed with brine (25 mL), dried over MgSO4, filtered, and concentrated. The solid was purified by colunm chromatography on silica gel (5% -* 15% ethyl acetate in hexanes) to afford 2-bromo-4-nitrobenzaldehyde (1 .41 g, 9% yield) as a colorless fluffy solid: ‘H NMR (400 MHz, CDC13) ö 10.41 (s, 1 H), 8.51 (d, J=2.0 Hz, 1 H),8.25 (dd, J=8.4, 2.1 Hz, 1 H), 8.06 (d, J8.6 Hz, 1 H).
References:
BRISTOL-MYERS SQUIBB COMPANY;HARTZ, Richard A.;AHUJA, Vijay T.;BRONSON, Joanne J.;DZIERBA, Carolyn Diane;MACOR, John E.;NARA, Susheel Jethanand;RAJAMANI, Ramkumar WO2015/6100, 2015, A1 Location in patent:Page/Page column 75; 76
1313762-48-9
2 suppliers
inquiry

5274-71-5
78 suppliers
$35.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5274-71-5
78 suppliers
$35.00/250mg
16426-64-5
164 suppliers
$5.00/250mg

5274-71-5
78 suppliers
$35.00/250mg
940-05-6
75 suppliers
inquiry

5274-71-5
78 suppliers
$35.00/250mg