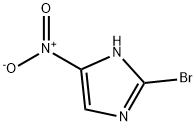
2-Bromo-4-nitroimidazole synthesis
- Product Name:2-Bromo-4-nitroimidazole
- CAS Number:65902-59-2
- Molecular formula:C3H2BrN3O2
- Molecular Weight:191.97
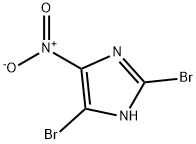
6154-30-9
68 suppliers
inquiry

65902-59-2
304 suppliers
$6.00/1g
Yield:65902-59-2 71%
Reaction Conditions:
Stage #1:2,4(5)-dibromo-5(4)-nitroimidazole with tetrabutylammonium borohydride in 1,4-dioxane at 20; for 23 h;Heating / reflux;
Stage #2: with hydrogenchloride in 1,4-dioxane;waterProduct distribution / selectivity;
Steps:
3 Synthesis of 2-bromo-4-nitroimidazole
Example 3 Synthesis of 2-bromo-4-nitroimidazole Into 1,4-dioxane (1 ml) solution of tetra-n-butylammonium borohydride (638 mg) was added dropwise 1,4-dioxane (1 ml) solution of 2,5-dibromo-4-nitroimidazole (89.5 mg) at a room temperature, after being refluxed the reaction mixture for 23 hours, excessive reagents were quenched by adding concentrated hydrochloric acid, then water and ethyl acetate were added. The organic layer was washed with an aqueous solution being saturated with sodium chloride, then dried over anhydrous sodium sulfate, and purified by a thin layer chromatography (developing agent: ethyl acetate) to obtain 2-bromo-4-nitroimidazole (44.9 g, yield: 71%) of white powdery product. 1H-NMR (DMSO-d6) δ(ppm): 8.42 (s, 1H), 14. 10 (bs, 1H).
References:
Otsuka Pharmaceutical Company, Limited EP1553088, 2005, A1 Location in patent:Page/Page column 49

6154-30-9
68 suppliers
inquiry

65902-59-2
304 suppliers
$6.00/1g
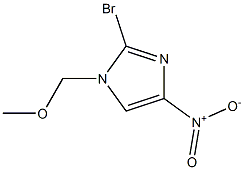
683276-47-3
0 suppliers
inquiry

65902-59-2
304 suppliers
$6.00/1g
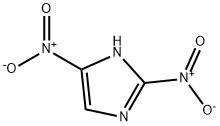
5213-49-0
69 suppliers
inquiry

65902-59-2
304 suppliers
$6.00/1g