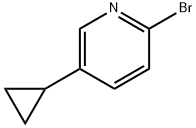
2-broMo-5-cyclopropylpyridine synthesis
- Product Name:2-broMo-5-cyclopropylpyridine
- CAS Number:1142197-14-5
- Molecular formula:C8H8BrN
- Molecular Weight:198.06
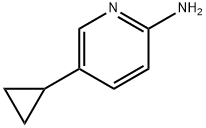
893738-68-6

1142197-14-5
General method: 5-cyclopropylpyridin-2-amine (1 mmol) was dissolved in dibromo(chloro)methane (6 mL) with the corresponding Cu(II) halide (0.5 mmol) at 25 °C and under argon protection. Alkyl nitrite (1.1 mmol) was added slowly and dropwise over 5 min, followed by stirring of the reaction mixture at a suitable temperature (see Tables 1 and 2) until GC-MS analysis showed complete consumption of the feedstock. Upon completion of the reaction, the mixture was poured into 1N NaOH solution (20 mL), stirred for 1 h, and then extracted with dichloromethane (3 x 20 mL). The organic phases were combined, dried with anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography using dichloromethane as eluent. 2-Bromo-5-cyclopropylpyridine (2a): 76% yield. Thin layer chromatography Rf value was 0.49 (unfolding agent: dichloromethane). NMR hydrogen spectrum (400MHz, CDCl3) δ 8.17 (d, J=2.4Hz, 1H), 7.34 (d, J=8.2Hz, 1H), 7.15 (dd, J=8.2,2.4Hz, 1H), 1.89-1.82 (m, 1H), 1.10-0.98 (m, 2H), 0.75-0.64 (m, 2H) . NMR carbon spectrum (100 MHz, CDCl3) δ 148.6,138.8,138.7,135.4,127.5,12.5,9.1. GC-MS analysis: retention time tR = 7.031 min, mass-to-charge ratio m/z (relative abundance) 197 (43.52%), 199 (41.85%).

893738-68-6
93 suppliers
$45.00/10mg

1142197-14-5
52 suppliers
$106.00/100mg
Yield: 76%
Reaction Conditions:
with copper(ll) bromide;isopentyl nitrite at 25; for 24 h;Inert atmosphere;Sandmeyer Reaction;Reagent/catalyst;
Steps:
General procedure for preparation of halogenopyridines 2, 3
General procedure: Aminopyridine (1 mmol) and corresponding Cu(II) halide (0.5 mmol) were dissolveddibromo(chloro)methane (6 mL) at 25 °C under an argon atmosphere. Alkyl nitrite (1.1 mmol)was added dropwise over 5 min and reaction mixture stirred at the appropriate temperature(Table 1 and 2) until GC-MS analysis of the reaction mixture indicated full consumption the of starting material. The reaction mixture was poured into 1 N NaOH (20 mL), stirred for 1 h andextracted with CH2Cl2 (3 × 20 mL). The combined organic extracts were dried (Na2SO4) andevaporated to dryness. The crude product was purified by column chromatography on silica gel,eluting with CH2Cl2. 2-Bromo-5-cyclopropylpyridine (2a): yield 76%. Rf = 0.49. 1H NMR (400MHz, CDCl3) δ 8.17 (d, J = 2.4 Hz, 1H), 7.34 (d, J = 8.2 Hz, 1H), 7.15 (dd, J =8.2, 2.4 Hz, 1H), 1.89 - 1.82 (m, 1H), 1.10 - 0.98 (m, 2H), 0.75 - 0.64 (m,2H). 13C NMR (100 MHz, CDCl3) δ 148.6, 138.8, 138.7, 135.4, 127.5, 12.5,9.1. GC-MS: tR= 7.031 min (m/z (rel. in.)) 197 (43.52%), 199 (41.85%).
References:
Striela, Romualdas;Urbelis, Gintaras;Sūdžius, Jurgis;Stončius, Sigitas;Sadzevičienė, Rita;Labanauskas, Linas [Tetrahedron Letters,2017,vol. 58,# 17,p. 1681 - 1683] Location in patent:supporting information
624-28-2
723 suppliers
$5.00/5g
411235-57-9
427 suppliers
$6.00/1g

1142197-14-5
52 suppliers
$106.00/100mg

67-56-1
791 suppliers
$7.29/5ml-f

893738-68-6
93 suppliers
$45.00/10mg
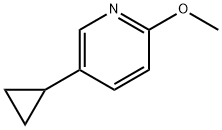
1063960-83-7
6 suppliers
inquiry

1142197-14-5
52 suppliers
$106.00/100mg
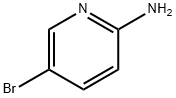
1072-97-5
707 suppliers
$9.00/1g

1142197-14-5
52 suppliers
$106.00/100mg

1072-97-5
707 suppliers
$9.00/1g

1063960-83-7
6 suppliers
inquiry

1142197-14-5
52 suppliers
$106.00/100mg