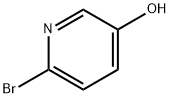
2-Bromo-5-hydroxypyridine synthesis
- Product Name:2-Bromo-5-hydroxypyridine
- CAS Number:55717-45-8
- Molecular formula:C5H4BrNO
- Molecular Weight:174
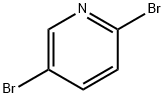
624-28-2

55717-45-8
To a 250 mL three-necked flask equipped with a mechanical stirrer, thermocouple, and nitrogen inlet, 2,5-dibromopyridine (9.98 g, 42.1 mmol) was added to 53 mL of anhydrous THF under a nitrogen atmosphere and dissolved to form a light tan solution. An ether solution (23 mL) of 2 M isopropylmagnesium chloride (i-PrMgCl) was slowly added via syringe over 3 min. The reaction mixture was transformed into a brown suspension when about 50% of Grignard reagent was added. The addition of i-PrMgCl triggered an exothermic reaction and the temperature was raised to 36 °C. After 90 min of continuous stirring, the suspension was cooled to 2 °C and trimethylborate (B(OMe)3) was rapidly added via syringe. The reaction was exothermic to 6 °C, followed by removal of the ice bath. After stirring overnight, glacial acetic acid (3.79 g) was added to dissolve all solids to form a dark brown solution. The solution was cooled in an ice bath and 5.25 g of 30% hydrogen peroxide solution was slowly added dropwise, with the rate of dropwise acceleration controlled to keep the reaction temperature from exceeding 12 °C. The reaction was then heated to 6 °C with stirring overnight. After the dropwise addition, stirring was continued for 90 minutes, followed by the addition of ether (150 mL) and water (100 mL) for extraction. The aqueous layer was separated and further extracted with ether (2 x 100 mL). The organic phases were combined and washed sequentially with 100 mL of 10% sodium bisulfite solution and brine. The organic phase was dried over anhydrous magnesium sulfate (MgSO4) and concentrated by rotary evaporation to a brown oil, which solidified on standing to give a brown solid (7.95 g). The crude product was adsorbed on 15 g Celite and purified by rapid column chromatography using a 220 g silica gel column with hexane/ethyl acetate gradient elution. The target fraction was collected and evaporation of the solvent gave 4.81 g of off-white solid product in 66% yield. The nuclear magnetic resonance (NMR) spectrum of the product was consistent with that of the 6-bromo-3-pyridinol standard.1H NMR (DMSO-d6, 400 MHz) δ 10.24 (s, 1H), 7.94 (d, J = 3.0 Hz, 1H), 7.42 (d, J = 8.6 Hz, 1H), 7.17 (dd, J = 3.0, 8.6 Hz, 1H); 13C NMR (DMSO-d6, 101 MHz) δ 153.74, 138.13, 129.30, 128.14, 126.21.
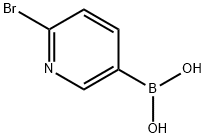
223463-14-7
247 suppliers
$6.00/250mg

55717-45-8
288 suppliers
$5.00/250mg
Yield:55717-45-8 88%
Reaction Conditions:
Stage #1: 6-bromopyridin-3-ylboronic acidwith dihydrogen peroxide;acetic acid in tetrahydrofuran at 0 - 20;
Stage #2: with sodium hydrogencarbonate in tetrahydrofuran;water;
Steps:
99.1
Step 1; 2-Bromo-pyridin-5-ol:; To a solution of 6-bromopyridin-3-ylboronic acid (9.5 g, 43.48 mmol) in THF (180 mL) was added oxydol (8.8 g, 98.35 mmol) dropwise with stirring at 0 0C. After 10 minutes, acetic acid (5.6 g, 93.33 mmol) was added dropwise with stirring at O 0C. The resulting solution was stirred overnight at room temperature. The product was precipitated after addition OfNaHSO3 and NaHCO3. The resulting solution was extracted with EtOAc (3 x 80 mL) and the organic layers were combined and dried over MgSO4, The solvent was concentrated to give 7 g (88%) of 2-bromo-pyridin-5-ol.
References:
WO2006/55187,2006,A1 Location in patent:Page/Page column 90

624-28-2
720 suppliers
$5.00/5g

55717-45-8
288 suppliers
$5.00/250mg
13534-97-9
437 suppliers
$6.00/1g

55717-45-8
288 suppliers
$5.00/250mg
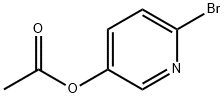
186593-28-2
6 suppliers
inquiry

55717-45-8
288 suppliers
$5.00/250mg
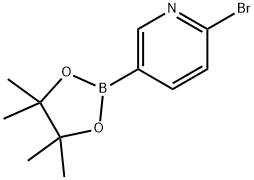
214360-62-0
163 suppliers
$9.00/250mg

55717-45-8
288 suppliers
$5.00/250mg