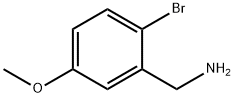
2-BROMO-5-METHOXYBENZYLAMINE synthesis
- Product Name:2-BROMO-5-METHOXYBENZYLAMINE
- CAS Number:887581-09-1
- Molecular formula:C8H10BrNO
- Molecular Weight:216.08
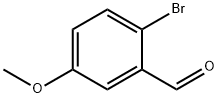
7507-86-0
194 suppliers
$7.00/1g

887581-09-1
32 suppliers
inquiry
Yield:887581-09-1 95%
Reaction Conditions:
Stage #1: 2-bromo-5-methoxy-benzaldehydewith tert-butyldimethylsilane;methyl carbamate;trifluoroacetic acid in acetonitrile at 80; for 6 h;
Stage #2: with water;lithium hydroxide in tetrahydrofuran;methanol at 80; for 16 h;
Steps:
2-Bromo-5-methoxybenzylamine (3b).
To a stirred solution of bromobenzaldehyde 1b (1.0 g, 4.6 mmol) and methyl carbamate (524 mg, 7.0 mmol) in acetonitrile (12 mL), were added sequentially TFA (0.71 mL, 9.3 mmol) and tert-butyldiemthylsilane (TBDMSH) (1.53 mL, 9.3 mmol) and the resulting solution was stirred at 80 °C for 6 h. The reaction mixture was concentrated in vacuo and the residue dissolved in a mixture of THF, MeOH and aq. LiOH [1.95 g, 46.5 mmol in H2O (5 mL)] (1:1:1, total 15 mL) and heated at 80 °C for 16 h. The reaction mixture was cooled to RT and then treated with aqueous NaOH [700 mg in H2O (10 mL)] and the mixture was extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with saturated NaCl solution, dried (Na2SO4), and filtered. Evaporation of the solvent and purification of the crude material by flash chromatography (short column, ethyl acetate/hexane, 1:1 to 100% ethyl acetate, then 5:95 methanol/ethyl acetate to 10:90 methanol/ethyl acetate) furnished the benzylamine 3b (960 mg, 95%) as pale yellowish viscous liquid. 1H NMR (400 MHz, CDCl3): = 7.40 (d, J = 8.6 Hz, 1H, Ar-H), 6.93 (d, J = 3.0 Hz, 1H, Ar-H), 6.67 (dd, J = 8.6, 3.0 Hz, 1H, Ar-H), 3.86 (s, 2H, CH2NH2), 3.78 (s, 3H, OCH3), 2.12 (br. s, 2H, CH2NH2); 13C NMR (100 MHz, CDCl3): = 159.3 (C), 142.5 (C), 133.4 (CH), 114.8 (CH), 114.1 (CH), 113.7 (C), 55.4 (OCH3), 46.8 (CH2NH2); HRMS (ESI) calcd for C8H11BrNO [M+H]+ 216.0018, found 216.0017.
References:
Satyanarayana, Gedu;Maier, Martin E. [Tetrahedron,2012,vol. 68,# 6,p. 1745 - 1749] Location in patent:supporting information; experimental part
138642-47-4
110 suppliers
$7.00/250mg

887581-09-1
32 suppliers
inquiry
99848-43-8
19 suppliers
$45.00/5mg

887581-09-1
32 suppliers
inquiry
19614-12-1
130 suppliers
$6.00/1g

887581-09-1
32 suppliers
inquiry