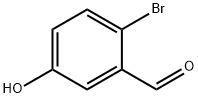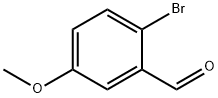
2-Bromo-5-methoxybenzaldehyde synthesis
- Product Name:2-Bromo-5-methoxybenzaldehyde
- CAS Number:7507-86-0
- Molecular formula:C8H7BrO2
- Molecular Weight:215.04
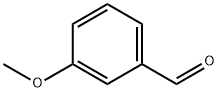
591-31-1

7507-86-0
3-Methoxybenzaldehyde (6.06 g, 44.6 mmol, 1.0 eq.) was used as a starting material, which was dissolved in acetic acid (10 mL), followed by slow dropwise addition of bromine (2.75 mL, 53.6 mmol, 1.2 eq.). The reaction mixture was stirred at 25°C for 36 hours. Upon completion of the reaction, the reaction was quenched with saturated sodium sulfite solution (50 mL), the mixture was poured into water (20 mL) and extracted with ether (5 x 30 mL). The organic layers were combined, washed sequentially with water (3 x 25 mL) and brine (30 mL), dried over anhydrous magnesium sulfate and concentrated to give the target product 2-bromo-5-methoxybenzaldehyde (9.49 g, 99% yield) as a dark yellow solid. Alternatively, the aldehyde was commercially available. Next, 2-bromo-5-methoxybenzaldehyde (9.49 g, 44.2 mmol, 1.0 eq.) was dissolved in acetonitrile (200 mL), and this solution was added to stirring phosphonate (14.9 g, 44.2 mmol, 1.0 eq.), lithium chloride (2.44 g, 57.4 mmol, 1.3 eq.), and 1,8-diazabicyclo[5.4. 0]undec-7-ene (DBU, 6.59 mL, 44.2 mmol, 1.0 equiv) in a mixture. The resulting dark yellow solution was stirred at 25°C for 12 hours. Upon completion of the reaction, the reaction was quenched by addition of saturated ammonium chloride solution (100 mL) and concentrated to remove the organic solvent. The residue was redissolved in ethyl acetate (50 mL), poured into water (50 mL) and extracted with ethyl acetate (3 x 50 mL). The organic extracts were combined, washed sequentially with water (50 mL) and brine (50 mL), dried over anhydrous magnesium sulfate and concentrated to give the diester product (17.5 g, 99% yield) as a yellow oil. The product was characterized as follows: rf = 0.57 (silica gel plate, hexane/ethyl acetate, 4:1); IR (thin film) 2979, 2936, 1724, 1643, 1590, 1569, 1465, 1285, 1238, 1194, 1156, 1097, 1019 cm^-1; ^1H NMR (400 MHz, CDCl3) δ 7.80 (s, 1H), 7.47 (d, J = 8.8 Hz, 1H), 6.94 (d, J = 2.8 Hz, 1H), 6.77 (dd, J = 8.8, 2.8 Hz, 1H), 4.29 (q, J = 7.2 Hz, 2H), 3.76 (s, 3H), 3.33 (s, 2H), 1.45 (s, 9H), 1.35 (t, J = 7.2 Hz, 3H); ^13C NMR (75 MHz, CDCl3) δ 170.2, 166.9, 158.7, 140.6, 136.3, 133.3, 128.3, 116.5, 115.0, 114.3, 81.1, 61.2, 55.5, 35.2, 28.0, 14.2; HRMS (MALDI-FTMS) calculated value C18H23BrO5 [M+] 398.0729, measured value 398.0746.
Yield:7507-86-0 100%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 60 h;
Steps:
AH.1
To a solution of 2-bromo-5-hydroxybenzaldehyde (1000.0 mg, 4.975 mmol) in DMF (20 mL) were added methyl iodide (0.31 mL, 5.000 mmol) and potassium carbonate (1036.6 mg, 7.500 mmol). The reaction mixture was stirred at rt for 2.5 days. Water was added and the reaction mixture was extracted with hexanes-ether (1:1) solvent system. The combined organic phases were dried over Na2SO4, filtered and concentrated in vacuum to yield 2-bromo-5-methoxybenzaldehyde (1069.8 mg, 100%) as colorless oil. LC-MS (M+H)+=215.11. 1H NMR (500 MHz, DMSO-d6) δ 10.18 (s, 1H). 7.70 (d, J=10 Hz, 1H) 7.35 (d, J=3 Hz, 1H) 7.23 (dt, J1=7.0 Hz, J2=2.0 Hz, 1H) 3.83 (s, 3H).
References:
US2008/194535,2008,A1 Location in patent:Page/Page column 30

591-31-1
430 suppliers
$10.00/5g

7507-86-0
192 suppliers
$7.00/1g
150192-39-5
67 suppliers
$42.00/1g

7507-86-0
192 suppliers
$7.00/1g
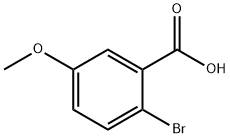
22921-68-2
279 suppliers
$6.00/5g

7507-86-0
192 suppliers
$7.00/1g
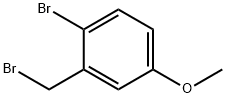
19614-12-1
133 suppliers
$6.00/1g

7507-86-0
192 suppliers
$7.00/1g