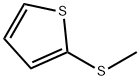
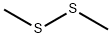2-broMo-5-(Methylthio)thiophene synthesis
- Product Name:2-broMo-5-(Methylthio)thiophene
- CAS Number:86369-96-2
- Molecular formula:C5H5BrS2
- Molecular Weight:209.13
Yield:86369-96-2 100%
Reaction Conditions:
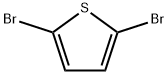
Stage #1: 2,5-dibromothiophenwith n-butyllithium in hexanes;diethyl ether at -78 - -65;
Stage #2: Dimethyldisulphide in hexanes;diethyl ether at 0 - 20;
Steps:
7.A Example 7. Preparation of N-hydroxy-4-({5-[5-(3, 3,4, 4,4- pentafluorobutyl) PYRIDIN-2-YLLTHIEN-2-YL} SULFONYL) tetrahydro-2H-pyran-4- carboxamide hydrochloride; Part A. Preparation of 2-bromo-5- (methylthio) thiophene
2,5-Dibromothiophene (from Aldrich, 40.0 g, MW 241.93) was dissolved in diethyl ether (300 ml) and then cooled TO-78°C. A solution of n-butyl lithium (from Aldrich, 1.6 M in hexanes, 118 ml, 1.15 eq) was slowly added while maintaining the temperature at less than - 65°C. After complete mono-exchange, a solution of dimethyldisulfide (from Aldrich, 14.2 ml, MW 94.20, 1.0 eq) in diethyl ether (20 ml) was added and the ice bath was removed while stirring, allowing the mixture to warm to ambient temperature. After the addition was complete, the mixture was diluted with water (500 ml) and then separated. The organic layer was washed with water (2x200 ml), washed with brine (1X200 ml), dried over sodium sulfate, and concentrated to form a black residue. The residue was passed through a silica gel plug and eluted with hexanes. Evaporation of the organics afforded the desired compound as a tan oil (34.3 g, 100+% yield). Some non-substituted thiophene was produced during the reaction and co-eluted with the PRODUCT. 1H NMR confirmed the presence of the desired compound. The"equivalents"above indicate equivalents relative to the charged amount of 2, 5-dibromothiophene.
References:
WO2004/48368,2004,A2 Location in patent:Page 132; 133
5780-36-9
108 suppliers
$28.60/25mg

86369-96-2
8 suppliers
inquiry