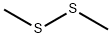
Dimethyl disulfide synthesis
- Product Name:Dimethyl disulfide
- CAS Number:624-92-0
- Molecular formula:C2H6S2
- Molecular Weight:94.2
In industry,dimethyl sulfate method is adopted to synthesize dimethyl disulfide.
Na2S+S→Na2S2Na2S2+(CH3)2SO4→CH3SSCH3+Na2SO4
From magnesium methyl iodide and S2Cl2, or from S2S2 and sodium methyl sulfate; also from methyl bromide and sodium thiosulfate, after which the resulting sodium methylthiosulfate is heated to yield dimethyl disulfide.
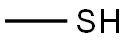
74-93-1
143 suppliers
$454.02/5MG
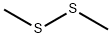
624-92-0
352 suppliers
$26.39/5ml
Yield:624-92-0 97.6%
Reaction Conditions:
Stage #1: methylthiolwith sodium hydroxide at 51;
Stage #2: with oxygen at 55; under 4500.45 Torr;Temperature;Pressure;Reagent/catalyst;
Steps:
6
(1) liquid alkali absorption: Methyl mercaptan is directly gasified by a primary static mixer, with the excess sodium hydroxide solution to absorb the reaction, the unabsorbed methyl mercaptan gas by the secondary static mixer and the excess sodium hydroxide solution to absorb the reaction again; The reaction temperature was 51 ° C, the mass percentage of sodium hydroxide solution was 18.5%, and the amount of sodium hydroxide was 4% by mole.The sodium methanethiol product obtained by the reaction in this step contains, in mass percent, 22% sodium methanethiol, 3.0% sodium hydroxide, the balance being water, and trace amounts of other impurities,And the yield of sodium methanethiolate is as high as 100%. (2) oxidation: The oxidation reaction of the sodium methanethiolate product obtained in step (1) with air and powdered sulfonated cobalt phthalocyanine as catalyst is carried out. Among them, the control reaction pressure .6Mpa, reaction temperature 55 ° C, stirring rate of 1000r / min, the amount of catalyst added to the mass of sodium methanilate 2 5ppm, reaction plus holding time of 11 Om i η;After the oxidation reaction step, the conversion of sodium methanethiol was as high as 98.7%, and the product of the dimethyl disulfide product prepared by the oxidation reaction was a colorless transparent liquid and no floc, and the product was briefly filtered , And then detected that: its dimethyl disulfide yield that is as high as 97.6%, purity up to 99.8%, can be used directly.
References:
CN105924372,2016,A Location in patent:Paragraph 0038-0040

67-56-1
790 suppliers
$9.00/25ml
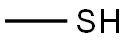
74-93-1
143 suppliers
$454.02/5MG
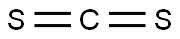
75-15-0
273 suppliers
$40.00/100g
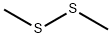
624-92-0
352 suppliers
$26.39/5ml
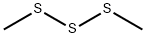
3658-80-8
269 suppliers
$34.00/5g
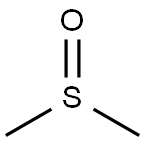
67-68-5
1328 suppliers
$15.00/100g
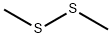
624-92-0
352 suppliers
$26.39/5ml

124-63-0
0 suppliers
$10.00/5g
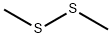
624-92-0
352 suppliers
$26.39/5ml
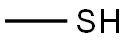
74-93-1
143 suppliers
$454.02/5MG
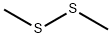
624-92-0
352 suppliers
$26.39/5ml
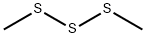
3658-80-8
269 suppliers
$34.00/5g
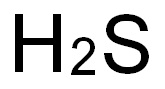
7783-06-4
77 suppliers
$158.00/25ml