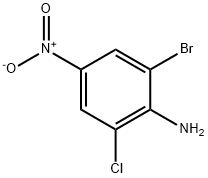
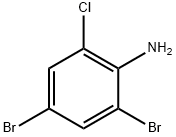
2-BROMO-6-CHLORO-4-NITROANILINE synthesis
- Product Name:2-BROMO-6-CHLORO-4-NITROANILINE
- CAS Number:99-29-6
- Molecular formula:C6H4BrClN2O2
- Molecular Weight:251.47
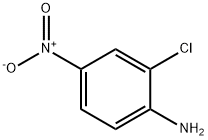
121-87-9

99-29-6
General procedure for the synthesis of 2-chloro-4-nitro-6-bromoaniline from 2-chloro-4-nitroaniline: 345 mg (2 mmol) of 2-chloro-4-nitroaniline and 143 mg (1.2 mmol) of potassium bromide were dissolved in 10 mL of a solvent mixture of acetic acid (AcOH) and water (AcOH:H2O = 9:1), and transferred to a 50 mL three-neck flask. The reaction system was placed in a thermostatic magnetic stirring water bath and the reaction temperature was controlled at 50 °C. The reaction mixture was stirred at 30 °C for 1 h. 1.8 g (1.8 mmol) of ZnAl-BrO3-LDHs was added slowly in batches over 15 min before the reaction started. after the reaction was completed, the reaction solution was extracted with dichloromethane and the organic phases were combined. Silica gel (200-300 mesh) was added to the organic phase for adsorption, followed by distillation under reduced pressure to remove the dichloromethane. The residue was purified by column chromatography using a solvent mixture of petroleum ether and ethyl acetate (petroleum ether:ethyl acetate= 10:1) as eluent to give 478 mg of pure product. The product was a yellow solid in 95% yield.

121-87-9
27 suppliers
$35.79/250g

99-29-6
153 suppliers
$8.00/10g
Yield: 97.62%
Reaction Conditions:
with sodium chlorate;sulfuric acid;hydrogen bromide in water at 35 - 65;
Steps:
1.d; 2.d; 3.d; 1.d; 2.d; 3.d; 4.d; 5-10
(d) Bromination reaction: add 60g sulfuric acid and 240g water to 450g of brominated waste acid to prepare bottom acid, add 90g of o-chloro-p-nitroaniline prepared in step (c) to bottom acid and add hydrobromic acid 44.2g, prepare 19.5g of sodium chlorate as a solution, slowly drop in, dropwise addition temperature 35 , drop to warm up to 65 heat preservation reaction to the end, suction filtration and washing to obtain 2-chloro-4-nitro-6- Bromo-aniline 127.7g, purity 99.1%.
References:
Zhejiang Runtu Institute Co., Ltd.;Gu Gaowei;Chen Baoxing;Gao Lijiang;Huang Zhong CN111039798, 2020, A Location in patent:Paragraph 0040; 0044; 0046; 0050-0051; 0055-0056; 0060-0061
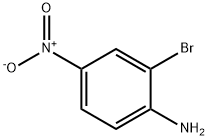
13296-94-1
178 suppliers
$6.00/1g

99-29-6
153 suppliers
$8.00/10g
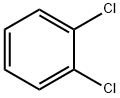
95-50-1
601 suppliers
$10.00/5g

99-29-6
153 suppliers
$8.00/10g
874-18-0
79 suppliers
$23.19/5g

99-29-6
153 suppliers
$8.00/10g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

99-29-6
153 suppliers
$8.00/10g